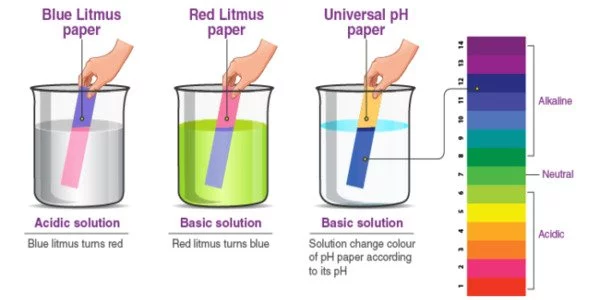
Litmus is a water-soluble mixture of various dyes extracted from lichens. It is a lichen-derived coloring matter that turns red in acid solutions and blue in alkaline solutions and is used as an acid-base indicator. It is frequently absorbed onto filter paper to produce one of the oldest forms of pH indicator, which is used to test materials for acidity. Litmus, which turns red in acidic solutions and blue in alkaline solutions, is the oldest and most widely used indicator of whether a substance is an acid or a base.
Litmus is most commonly used to determine whether a solution is acidic or basic. The color transition occurs at 25 °C (77 °F) over the pH range 4.5-8.3, with light blue litmus paper turning red in acidic conditions and red litmus paper turning blue in basic or alkaline conditions. Purple litmus paper is considered neutral.
History
The term “litmus” is derived from the Norse word for “dye” or “color.” Arnaldus de Villa Nova, a Spanish physician, used litmus for the first time around 1300. Blue dye was extracted from lichens beginning in the 16th century, particularly in the Netherlands.
The various colored components of litmus are produced by treating lichens with ammonia, potash, and lime in the presence of air. Litmus had been partially separated into azolitmin, erythrolitmin, spaniolitmin, and erythrolein by 1840. These appear to be mixtures of compounds that were discovered in 1961 as derivatives of the heterocyclic compound phenoxazine.
Litmus can be found in a variety of lichen species. Roccella tinctoria (South America), Roccella fuciformis (Angola and Madagascar), Roccella pygmaea (Algeria), Roccella phycopsis, Lecanora tartarea (Norway, Sweden), Variolaria dealbata, Ochrolechia parella, Parmotrema tinctorum, and Parmelia are used to make the dyes. The primary sources are currently Roccella montagnei (Mozambique) and Dendrographa leucophoea (California).
Uses
Litmus is most commonly used to determine whether a solution is acidic or basic because blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic or alkaline conditions, with the color change occurring over the pH range 4.5-8.3 at 25 °C (77 °F). Purple is the color of neutral litmus paper. Wet litmus paper can also be used to detect water-soluble gases that affect acidity or basicity; the gas dissolves in water, and the resulting solution colors the litmus paper. For example, alkaline ammonia gas turns red litmus paper blue. While all litmus paper functions as pH paper, the reverse is not true.
Litmus can also be prepared as an aqueous solution, which works in the same way. The solution is red under acidic conditions and blue under alkaline conditions. Litmus paper is filter paper that has been treated with a natural-soluble dye derived from lichen. Litmus paper is a type of paper that produces a result that can be used to determine the pH of a solution.