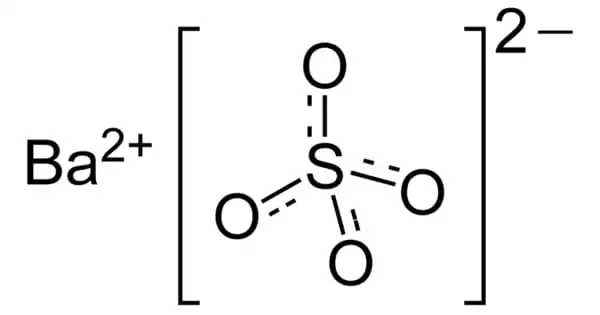
The inorganic compound barium sulfate has the chemical formula BaSO4. It appears as odorless white or yellowish powder or tiny crystals. It is a white crystalline substance that is odorless and water-insoluble. It is soluble in hot concentrated sulfuric acid and dilutes acids, alcohol, and is soluble in hot concentrated sulfuric acid. It is the barium sulfate salt, an alkaline, divalent metal.
It occurs naturally as the mineral barite, which is the primary commercial source of barium and products derived from it. The white opaque look and high density of the material are used in its primary applications. It is most commonly employed as a component in oil well drilling fluid, and it occurs naturally as the mineral barite.
Properties
BaSO4 is a sulfate salt of barium and it can be found in the form of mineral barite. It is a crystalline solid white which is insoluble in water and alcohol but soluble in concentrated acids. It is odorless. It is widely used in the production of oil and natural gas to get high-density drilling fluids by keeping the boreholes free of rock.
- Molecular Weight/ Molar Mass: 233.38 g/mol
- Density: 4.5 g/cm³
- Boiling Point: 1,600 °C
- Melting Point: 1,580 °C

Preparation
After mining and processing, commercial levels of barium sulfate are found in the mineral barite. To make impure barite, heat it with coke, also known as carbon, to generate water-soluble barium sulfide (BaS), which is then separated from the filths and treated with sulfuric acid to yield the pure barium sulfate product.
BaSO4 + 4 C → BaS + 4 CO
BaS + H2SO4 → BaSO + H2S
A second approach for obtaining pure barium sulfate is to start a reaction using sulphuric acid and barium carbonate or barium chloride.
Because it has a high lattice enthalpy and its hydration enthalpy drops faster than its lattice enthalpy when dissolved in water, barium sulphate is insoluble in water. In order for a chemical to be soluble, its lattice enthalpy must be low and fall faster than its hydration enthalpy.
Production
Almost all of the barium consumed commercially is obtained from barite, which is often highly impure. Barite is processed by thermo-chemical sulfate reduction (TSR), also known as carbothermal reduction (heating with coke) to give barium sulfide:
BaSO4 + 4 C → BaS + 4 CO
In contrast to barium sulfate, barium sulfide is soluble in water and readily converted to the oxide, carbonate, and halides. To produce highly pure barium sulfate, the sulfide or chloride is treated with sulfuric acid or sulfate salts:
BaS + H2SO4 → BaSO4 + H2S
This type of barium sulfate is known as blanc fixe, which translates to “permanent white” in French. Blanc fixe barium is the type of barium found in consumer products such as paints.
Barium sulfate is created in the laboratory by mixing solutions of barium ions and sulfate salts. Due to the insolubility of barium sulfate, the least hazardous salt of barium, wastes containing barium salts are occasionally treated with sodium sulfate to immobilize (detoxify) the barium. One of the most insoluble salts of sulfate is barium sulfate. Its limited solubility is used as a test for Ba2+ ions as well as sulfate in qualitative inorganic analysis.
Untreated raw materials such as natural baryte formed under hydrothermal conditions may contain many impurities, a.o., quartz, or even amorphous silica.
Occurrence
Barium sulfate occurs naturally as the mineral barite, which is widely found and used as the major source of barium and other barium compounds.
Barium Sulfate Uses
- It is used in the casting of copper anode plates as a coating material.
- It is used as a radiopaque agent.
- It is used as a component in oil well drilling fluids.
- It is used to increase the density of the polymer by acting as a filler for plastics.
- It is used in imaging of the GI tract during barium meal.
- It is used to diagnose certain disorders of the intestines, stomach, or esophagus.
Health effects/safety hazards
Due to its insoluble nature in aqueous fluids, barium sulfate is considered harmless and suitable for medicinal use. However, exposure to its dust or inhalation in high amounts can cause irritation to the eyes, nose, and respiratory system.