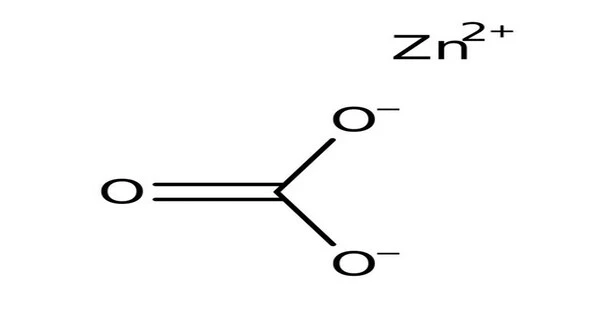
Zinc carbonate, abbreviated ZnCO3, is an inorganic compound. It is a type of organooxygen compound. It has the appearance of a white crystalline solid or powder that is insoluble in water. It is a white solid that is water insoluble. Smithsonite is a mineral that exists in nature. It is used in pharmaceuticals, the production of other zinc compounds, and as a feed additive.
It is made by reacting cold zinc sulfate solutions with potassium bicarbonate. The primary risk is the threat to the environment. When heated, it transforms into basic zinc carbonate [Zn5(CO3)2(OH)6]. Immediate action should be taken to limit its environmental spread.
Properties
Zinc carbonate is a white crystalline powder found in nature in the form of granular or earthy masses. It is also known as smithsonite, calamine, or zinc spar. It is colorless and transparent in its pure form, but it is frequently colored by the presence of iron, manganese, copper, and other elements.
- Chemical formula: ZnCO3
- Molar mass: 125.4
- Appearance: white solid
- Density: 4.434 g/cm3
- Melting point: 140 °C (284 °F; 413 K) (decomposes)
- Solubility in water: 0.91 mg/L
- Solubility product (Ksp): 1.46×10−10
Zinc carbonate adopts the same structure as calcium carbonate. Zinc is octahedral and each carbonate is bonded to six Zn centers such that oxygen atoms are three-coordinate.

Chemical Properties
Zinc carbonate reacts with acids like hydrochloric acid to form zinc chloride and releases carbon dioxide.
ZnCO3 + 2HCl → ZnCl2 + CO2 + H2O
Zinc carbonate undergoes decomposition forming zinc oxide and carbon dioxide. The chemical reaction is as below.
ZnCO3 → ZnO + CO2
Because of its basic nature, zinc carbonate dissolves easily in acidic solutions but is insoluble in water. Carbon dioxide is produced as a byproduct of the reaction. In an extremely strong base, this will also dissolve to form zincates. Zinc oxide can be formed by the thermal decomposition of zinc carbonate.
Production
In general, it forms as a secondary mineral in zinc mining deposits; for zinc carbonate to form, these deposits must contain oxidation zones. It can also be found in sedimentary deposits and in products that have been directly oxidized.
It’s made by grinding the Smithsonite mineral, formerly known as zinc spar. It can also be made by combining a sodium carbonate solution with a zinc salt like zinc sulfate. Sodium sulfate is still dissolved, and zinc carbonate forms:
ZnSO4 + Na2CO3 → ZnCO3 + Na2SO4
Uses
Zinc carbonate is usually used as a barrier against iron and steel which protects against dilapidation. It can be used on materials, pigments for painting, and agricultural stuff. This is also present in zinc anodes known as batteries used on appliances, television monitors, and lighting.
- Used principally in ointments and takes the place of the former impure carbonate termed calamine.
- Used in dusting upon inflamed surfaces as an astringent and absorbent.
- Calamine is a very oil treatment for pruritus. This natural product is zinc carbonate with a small quantity of iron oxide.
Health hazard
Inhaling zinc fumes and particles, primarily in industrial processes, causes zinc intoxication, while oral ingestion results in an excess of zinc in dietary supplements. It causes stomach pains, vomiting, and bleeding.
When you breathe in zinc carbonate, you can be affected. Contact can irritate the skin and eyes. Zinc carbonate can also cause coughing and wheezing by irritating the nose and throat. Excessive exposure can harm the liver.