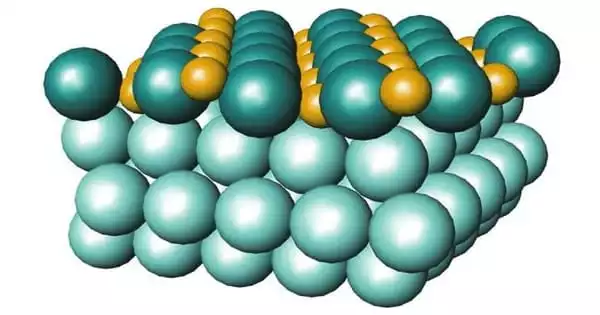
Iron oxychloride is the inorganic compound with the formula FeOCl. It typically refers to a compound containing iron (Fe), oxygen (O), and chlorine (Cl). This purple solid adopts a layered structure, akin to that of cadmium chloride. It is a layered compound with interesting properties, especially in catalysis, intercalation chemistry, and environmental remediation.
The material slowly hydrolyses in moist air. The solid intercalates electron donors such as tetrathiafulvalene and even pyridine to give mixed valence charge-transfer salts. Intercalation is accompanied by a marked increase in electrical conductivity and a color change to black.
Properties
- Chemical formula: ClFeO
- Molar mass: 107.29 g·mol−1
- Appearance: Vivid, dark violet, opaque crystals
- Solubility: Slightly soluble in water; hydrolyzes in moist air
- Thermal behavior: Decomposes upon heating
- Magnetism: Exhibits antiferromagnetic behavior at low temperatures
- Stability: Hygroscopic and sensitive to moisture; decomposes slowly in air over
Production
FeOCl is prepared by heating iron(III) oxide with ferric chloride at 370 °C (698 °F) over the course of several days:
Fe2O3 + FeCl3 → 3 FeOCl
Alternatively, FeOCl may be prepared by the thermal decomposition of FeCl3⋅6H2O at 220 °C (428 °F) over the course of one hour:
FeCl3 ⋅ 6H2O → FeOCl + 5 H2O + 2 HCl
Natural Occurrence
FeOCl occurs naturally as the rare mineral lepidocrocite when hydrated or in association with weathering products of iron-rich ores. However, pure FeOCl is rare in nature and more commonly encountered in synthetic form.
Applications
- Catalysis: Used as a catalyst in oxidative reactions and in dechlorination of pollutants.
- Environmental chemistry: Employed in photocatalytic degradation of organic pollutants.
- Battery research: Studied for its intercalation properties in lithium-ion battery electrodes.
- Materials science: Investigated as a layered compound for intercalation and magnetic studies.