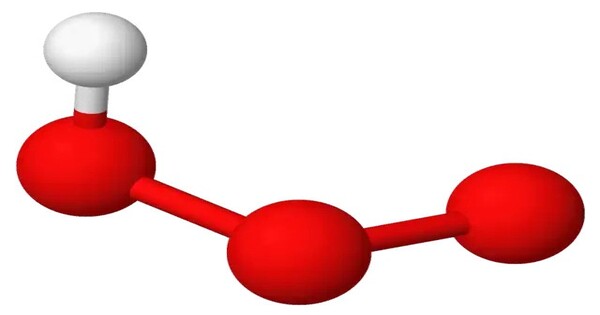
Hydrogen ozonide (HO3) is a radical molecule consisting of a hydrogen atom covalently bonded to an ozonide unit. It’s an unstable compound, and it can form in small quantities under certain conditions when ozone reacts with water. It is highly reactive and doesn’t exist for long periods because it quickly decomposes back into hydrogen peroxide and ozone.
It is possibly produced in the reaction of the hydroxyl radical with dioxygen: OH• + O2 → HO3•. It has been detected in a mass spectrometer experiment using HO+3 (protonated ozone) as precursor.
Properties
It is typically a colorless or slightly yellowish liquid. Because of its instability, it is difficult to isolate and handle in pure form. Its decomposition releases oxygen, similar to hydrogen peroxide’s behavior.
- Chemical formula: HO3
- Molar mass: 49.005 g·mol−1
- Instability: Hydrogen ozonide is highly unstable and decomposes rapidly into oxygen (O₂) and water (H₂O). The instability arises from the presence of both hydrogen peroxide and ozone, both of which are reactive themselves.
- Oxidizing Agent: It is a strong oxidizing agent. Due to the presence of ozone, hydrogen ozonide has the potential to oxidize various materials and compounds. This gives it applications in disinfection and water treatment.
Reactivity
It can decompose into oxygen gas, which might lead to violent reactions if not handled carefully. Its reactivity with other compounds can result in complex products depending on the environment.
Occurrences
Hydrogen ozonide does not occur naturally in large quantities, but it can be formed under specific conditions where ozone and hydrogen peroxide are present in close proximity, such as:
- Atmosphere: In certain parts of the atmosphere, especially during thunderstorms or in areas with high ozone concentrations, there could be brief formation of hydrogen ozonide as ozone reacts with moisture in the air.
- Water Treatment: In some advanced water purification processes, hydrogen ozonide may form as an intermediate product when ozone and hydrogen peroxide are used together to break down organic contaminants.
- Laboratory Formation: Hydrogen ozonide can be synthesized in laboratory settings by combining ozone and hydrogen peroxide under controlled conditions.
Because of its high instability and the difficulty of isolating it in a pure form, hydrogen ozonide isn’t something that’s commonly encountered outside of specific chemical processes. It’s mainly useful in situations requiring strong oxidation, such as certain forms of disinfection or wastewater treatment.