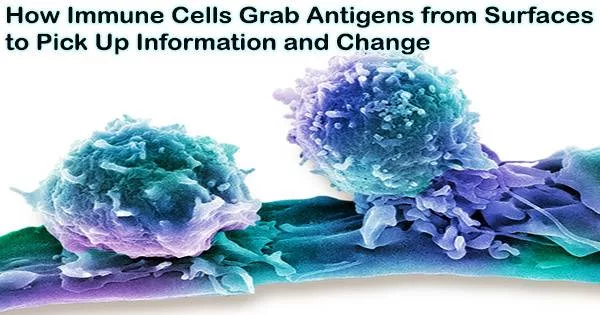
Most cells develop gradually, accumulating small adjustments that make them more environment-friendly. Immune cells evolve considerably more swiftly than other cells because they must immediately adapt to ward off new dangers. UCLA physicists now claim that part of it depends on their capacity to forcibly remove antigens from the surfaces of other cells and “study” them.
Immune cells, also known as white blood cells or leukocytes, are a crucial part of the body’s immune system. These cells are responsible for recognizing and destroying harmful pathogens, such as viruses, bacteria, and parasites, as well as cancer cells and other abnormal cells.
The B cells of the immune system, which produce antibodies that attack dangerous pathogens like viruses, bacteria, and parasites, are able to better assess the characteristics of specific antigens, compare which types of cells within the B cell population recognize and respond to each antigen most efficiently, and produce more of those cells by using this kind of mechanical force.
“The discovery that immune cells use mechanical force in addition to biochemical signaling in an active, deliberate way to advance their own evolution adds a new paradigm to studies of immune system learning and memory,” said Shenshen Wang, a UCLA assistant professor of physics and astronomy and corresponding author of the research.
“We showed that fast-evolving immune cells use tactile sense, by applying active tugging forces, to learn about their antigenic targets and to rank themselves,” Wang said. “Such active physical sensing allows the immune cell repertoire to respond effectively to current challenges while being plastic and adaptive against future threats.”
It has long been a tenet of biology that cells are capable of detecting and reacting to physical influences from the outside world. However, the discovery that they produce their own physical forces in order to acquire signals is recent, with implications for evolution that have not yet been fully investigated.
We are proposing a new paradigm of biological recognition via physical acquisition of stimuli, which may complement the current view centered on biochemical signaling. Meanwhile, it offers a fresh angle for understanding biological adaptation in light of physical influences on evolution. Our findings have broad implications for understanding biological learning and for physically steering adaptive evolution in general, and immune response in particular.
Professor Shenshen Wang
Wang said the findings may help scientists figure out how to guide the evolution of the immune system by designing a sequence of vaccines to help it learn to identify and encode the most important features of different antigens. For example, a pathogen that the immune system has never faced before could be recognized and neutralized considerably more swiftly by such a system.
The research is published in two journal papers. In Proceedings of the National Academy of Sciences, Wang and her co-author, UCLA physics doctoral student Hongda Jiang, describe how B cells pull antigens from the antigen-presenting cells to which they are tethered, generating mechanical stress that propagates through connected cell surfaces and changes the distribution of energy at the points where cells touch each other.
Wang and Jiang point out that the method still improves B cell learning even when B cells are unable to successfully extract an antigen from an antigen-presenting cell that is exceptionally stiff.
More significantly, the mechanical force’s deformation of the linked structure lengthens the time immune cells can “remember” what they have learnt and widens the variety of antigens they can recognize. Additionally, the detecting range increases noticeably if the pulling gets stronger over time.
In a paper accepted for publication in the open-access journal Physical Review X, Wang and Jiang address an immune cell dilemma: How the body’s finite set of immune B cells achieves a balance between responding with sufficient strength to antigens they’ve already encountered while also being able to recognize pathogens they’ve never seen before.
The latest findings demonstrate that mechanical force-driven evolution of B cells enhances memory variety, increasing the adaptability of natural immunity by balancing the potency of response to antigens with the breadth of coverage.
This ongoing process gives the body’s natural immune greater methods to adapt to new mutations and future variants by opening a wide array of biophysical routes to enhance antigen-recognition capacity.
The approach, according to the scientists, collaborates with biomechanical sensing and biochemical communication to create agile immune systems that can quickly develop to meet new challenges.
“We are proposing a new paradigm of biological recognition via physical acquisition of stimuli, which may complement the current view centered on biochemical signaling,” Wang said.
“Meanwhile, it offers a fresh angle for understanding biological adaptation in light of physical influences on evolution. Our findings have broad implications for understanding biological learning and for physically steering adaptive evolution in general, and immune response in particular.”