Several marine organisms, including mussels, secrete adhesive proteins that allow them to adhere to various surfaces in seawater. This appealing underwater adhesion property has sparked decades of research into the development of biomimetic glues for underwater repair or biological tissue repair. Existing glues, on the other hand, frequently lack desirable adhesion, are difficult to use underwater, or are not biocompatible for medical applications. Synthetic biology has provided a solution.
Researchers used synthetic biology to create a biocompatible adhesive that combines the best of spider silk and mussel foot protein. Researchers at Washington University in St. Louis’ McKelvey School of Engineering have developed a method that uses engineered microbes to produce the necessary ingredients for a biocompatible adhesive hydrogel that is as strong as spider silk and as adhesive as mussel foot protein (Mfp), which means it can stick to a variety of underwater surfaces.
Fuzhong Zhang, professor of energy, environmental, and chemical engineering, led the study, which was published in the journal ACS Applied Materials and Interfaces. “Researchers have been trying for quite some time to develop adhesives that can work underwater, or even just when they are wet,” said Eugene Kim, an assistant professor at George Mason University. Kim is the paper’s first author and worked on the project as a Ph.D. student in Zhang’s Washington University lab.
Researchers have been trying for quite some time to develop adhesives that can work underwater, or even just when they are wet. When underwater, we had to make sure the adhesive Mfp could remain on a surface during repair
Eugene Kim
Young-Shin Jun, professor of energy, environmental, and chemical engineering, and Guy Genin, Harold, and Kathleen Faught Professor of Mechanical Engineering were also members of the research team.
“We engineered microbes to produce a mussel foot protein (Mfp) and its oligomeric variants in a previous proof-of-concept study,” Kim explained. These variants are molecules composed of a repeating chain of Mfp with properties that differ depending on the number of repeats. “We wanted to see if synthetic biology could help with underwater adhesion, which is a difficult task for synthetic materials.”
Zhang’s lab demonstrated in 2018 that Mfp produced by engineered bacteria has similar underwater adhesive properties to natural Mfps and that they could create Mfp oligomers that are even stickier. Despite the fact that the microbial Mfp was extremely sticky, they were difficult to handle underwater because the protein molecules quickly diffused once added to water.
“When underwater, we had to make sure the adhesive Mfp could remain on a surface during repair,” Kim said.
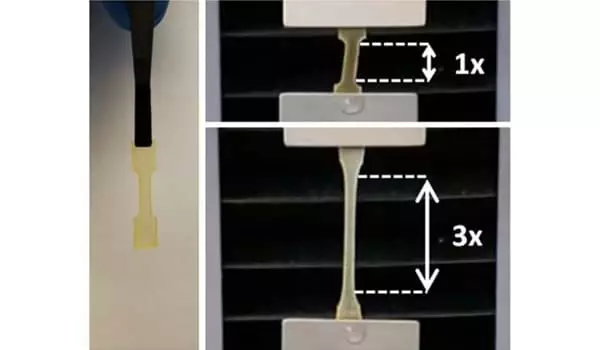
One popular method for preventing diffusion is to incorporate the adhesive Mfp protein into a hydrogel. The hydrogel must be strong, if not stronger than the adhesive force. However, it is extremely difficult to create a material that is both strong and adhesive, as there is frequently a trade-off between these two properties, according to Kim. “Many Mfp-inspired adhesives are ineffective. When used to adhere two surfaces underwater, the glue adheres to both surfaces but breaks apart, similar to separating an Oreo cookie and leaving the cream on both sides.”
That’s when spider silk came in handy. Zhang’s lab has also been using synthetic biology to engineer and produce spider silk proteins for many years. They created a silk-amyloid hybrid protein earlier this year that was stronger than steel and tougher than Kevlar. The high strength of this silk-amyloid hybrid, which keeps the material intact, was exactly what their adhesive required.
The researchers combined the silk-amyloid protein with Mfp and used a synthetic biology approach to create a tri-hybrid protein with the benefits of both Mfp’s strong adhesion and spider silk’s high strength. They created adhesive hydrogels using the tri-hybrid protein.
“We developed a design principle that allowed us to control the hydrogel’s cohesion and adhesion,” Zhang explained. “Because the gel is slightly denser than water, it can be used underwater, on or between two surfaces.”
The lab is particularly excited about the protein-based adhesive’s potential applications in tissue repair because it is biocompatible and biodegradable. This protein, they write in the paper, is especially appealing for tendon-bone repair, which currently has a high failure rate with suture-based strategies.
Jun observed, “Spiders, bacteria, slimy sea creatures, and rotator cuff tears have very little in common.” “It’s incredible that the Zhang lab was able to combine the best parts of the first three and create new elastic materials with molecular-scale crystalline structures that can serve as a stronger and more flexible adhesive. It will be even more exciting when we can use it in medical care to repair shoulder injuries.”
They can control the adhesion and strength of the hydrogel by controlling bacteria to modify each motif of the protein, including parts from spider silk and mussel foot proteins. This allows them to tailor it to meet the specific requirements for tendon-bone repair and other tissue repair needs. Genin contextualized the study within the context of humans’ long-standing, complicated relationships with bacteria.
“For the first time ever, we’ve gotten bacteria to help heal a wound,” Genin said. “For the first time, we’ve been able to hijack bacteria to make a material that’s unattainable any other way, with biomedical applications including rotator cuff surgeries that actually make limbs work again.”