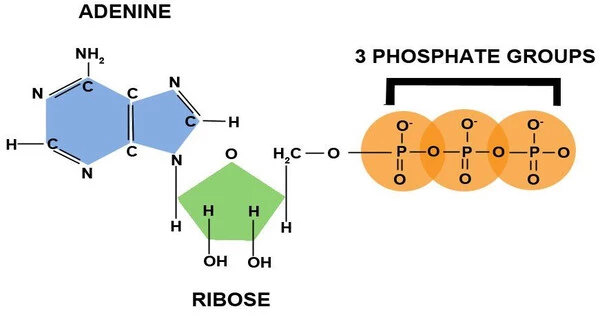
The storage of energy in molecules is an important aspect of biological processes, particularly in living organisms. Adenosine triphosphate (ATP) is the primary molecule involved in energy storage and transfer within cells. Adenine, ribose, and three phosphate groups combine to form ATP, a nucleotide. The bonds in ATP that connect the phosphate groups are high-energy bonds that store energy.
Molecular photoswitches that can convert and store energy could be used to improve the efficiency of solar energy harvesting. A group of scientists used quantum computing to identify a particularly efficient molecular structure for this purpose. As the team described in the journal Angewandte Chemie, their procedure was based on a dataset of more than 400,000 molecules, which they screened to find the optimum molecular structure for solar energy storage materials.
Solar energy is currently used either directly to generate electricity or indirectly through the energy stored in heat reservoirs. A third option would be to store solar energy in light-sensitive materials and then release it as needed. The EU-funded project MOST (“Molecular Solar Thermal Energy Storage”) is investigating molecules such as photoswitches that can absorb and store solar energy at room temperature in order to make completely emission-free solar energy utilization a reality.
The resulting chemical space consists of approximately 466,000 bicyclic dienes that we have screened for their potential applicability in MOST technology.
Kurt V. Mikkelsen
The research teams of Kurt V. Mikkelsen at the University of Copenhagen, (Denmark) and Kasper Moth-Poulsen at the Technical University of Catalonia, Barcelona (Spain), have taken a closer look at the photoswitches best suited for this task. They studied molecules known as bicyclic dienes, which switch to a high-energy state when illuminated. The most prominent example of this bicyclic diene system is known as norbornadiene quadricyclane, but a vast number of similar candidates exist. The researchers explain: “The resulting chemical space consists of approximately 466,000 bicyclic dienes that we have screened for their potential applicability in MOST technology.”
Machine learning is typically used to screen a database of this size, but this requires large amounts of training data based on real-world experiments, which the team lacked. The screening and evaluation of the database molecules using a previously developed algorithm and a novel evaluation score, “eta,” yielded a clear result: all six of the top scoring molecules differed from the original norbornadiene quadricyclane system at a critical point in the structure.
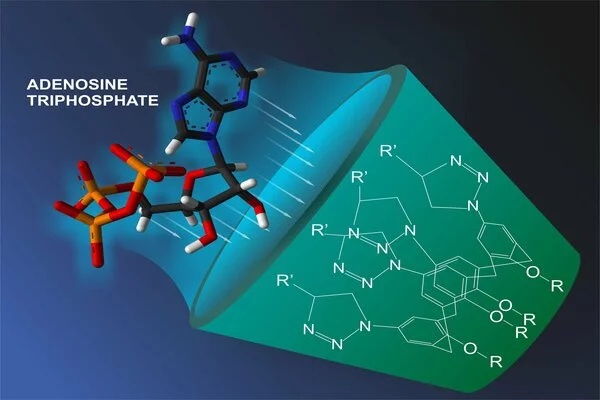
The researchers concluded that the structural change, which involved an expansion of the molecular bridge between the two carbon rings in the bicyclic part, enabled the new molecules to store more energy than the original norbornadiene.
The work of the researchers demonstrates the possibility of optimizing solar energy storage molecules. The new molecules, however, must first be synthesized and tested in real-world conditions. “Even though the systems can be synthetically prepared, there is no guarantee that they are soluble in relevant solvents and that they will actually photoswitch in high yield or at all, as we have assumed in eta,” the study’s authors state.
Despite this, the team created a new, large set of training data for machine learning algorithms, shortening the arduous research step prior to synthesis for future chemists tackling such systems. The authors see this much larger repository of bicyclic dienes coming into its own for research into photoswitches for a variety of applications, potentially making it easier to tailor molecules to specific needs.