Scientists have genetically created a mechanism to reverse pesticide resistance using CRISPR/Cas9 technology. The gene substitution technology provides a novel approach to combating the spread of fatal malaria while reducing the usage of insecticides that preserve key food crops.
Insecticides are critical in combating the global effect of mosquito-borne malaria and other infections, which kill an estimated 750,000 people each year. These insect-specific compounds, which cost more than $100 million to research and bring to market, are also essential for managing insect-driven agricultural damage, which threatens food security.
However, many insects have genetically changed in recent decades to become less susceptible to the efficacy of insecticides. In Africa, where long-lasting insecticide-treated bed nets and interior spraying are crucial interventions in the fight against malaria, many mosquito species have developed pesticide resistance, reducing the efficacy of these critical measures. Climate change is likely to exacerbate these issues in some locations.
Using CRISPR/Cas9 technology, biologists at the University of California, San Diego, have devised a mechanism for reversing pesticide resistance. According to Nature Communications, Tata Institute for Genetics and Society (TIGS) researchers Bhagyashree Kaduskar, Raja Kushwah, and Professor Ethan Bier used the genetic editing tool to replace an insecticide-resistant gene in fruit flies with the normal insecticide-susceptible form, a feat that could significantly reduce the amount of insecticides used.
An exciting option is to use allelic drives to generate novel variants of the VGSC that are even more susceptible to insecticides than wild-type VGSCs. This could potentially allow for the introduction of much lower doses of insecticides into the environment to control pests and disease vectors.
Craig Montell
“This technology could also be used to increase the proportion of a naturally occurring genetic variant in mosquitoes that renders them resistant to transmission or malarial parasites,” said Bier, senior author of the paper and professor of Cell and Developmental Biology in UC San Diego’s Division of Biological Sciences.
To distribute specific genes throughout a population, the researchers utilized a modified form of gene-drive, a method that uses CRISPR/Cas9 to break genomes at targeted places. When one parent transmits genetic elements to their offspring, the Cas9 protein slices the chromosome from the other parent at the corresponding place, and the genetic information is replicated into that region, ensuring that all offspring inherit the genetic trait. The new gene-drive contains an add-on that Bier and his colleagues previously created to bias the inheritance of simple genetic variants (also known as alleles) by simultaneously cutting an undesirable genetic variant (e.g., pesticide resistance) and replacing it with the preferred form (e.g., insecticide susceptible).
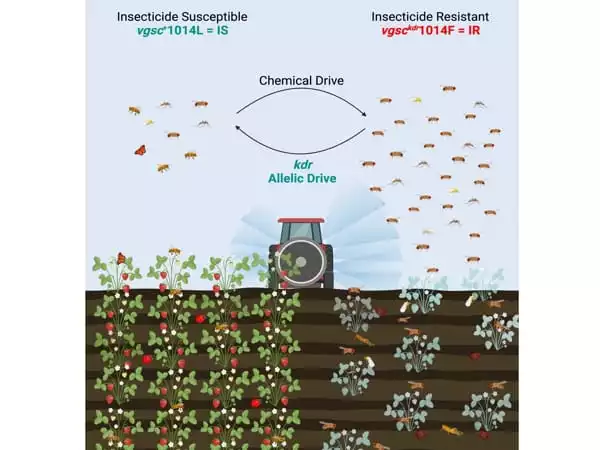
In the latest work, the researchers used this “allelic drive” technique to restore genetic sensitivity to insecticides, similar to insects in the wild before they evolved resistance. They concentrated on an insect protein known as the voltage-gated sodium channel (VGSC), which is a target for a common family of pesticides. Resistance to these insecticides, known as knockdown resistance, or “kdr,” is caused by mutations in the vgsc gene, which prevent the insecticide from binding to its VGSC protein target. The researchers switched a resistant kdr mutant with its regular natural counterpart, which is vulnerable to pesticides.
Starting with a population of 83 percent kdr (resistant) alleles and 17 percent normal alleles (insecticide susceptible), the allelic drive mechanism reversed that proportion to 13 percent resistant and 87 percent wild-type in ten generations. Bier also observes that insecticide resistance adaptations come at an evolutionary cost, rendering those insects less suitable in a Darwinian sense. According to him, combining the gene drive with the selective advantage of the more fit wild-type genetic variety resulted in a highly efficient and cooperative system.
Similar allelic drive mechanisms could be created in other insects, including mosquitos. This proof-of-principle adds a new way to pest- and vector-control toolboxes since it might be used in conjunction with other tactics to improve insecticide-based or parasite-reducing measures to reduce malaria spread.
“Through these allelic substitution tactics, it should be feasible to achieve the same level of pest control with significantly less insecticide use,” Bier added. “It should also be possible to create self-eliminating allelic drives, which are programmed to work very briefly in a population to enhance the relative frequency of a desired allele and then vanish. Such locally acting allelic drives might be reapplied as needed to boost the abundance of a naturally existing desirable trait, with the ultimate goal of removing all GMOs from the ecosystem.”
“An exciting option is to use allelic drives to generate novel variants of the VGSC that are even more susceptible to insecticides than wild-type VGSCs,” said co-author Craig Montell (UC Santa Barbara). “This could potentially allow for the introduction of much lower doses of insecticides into the environment to control pests and disease vectors.”