We frequently turn the audio down while travelling to unfamiliar places and paying attention to the road signs. What had been melody now sounds like noise and disrupts our concentration.
Noise has similarly complicated our understanding of how viral diseases like COVID affect human lungs. Data from patient lung tissues vary widely from patient to patient, making it difficult to understand the fundamental mechanisms by which SARS-Co-V2 initially infects lung cells. Additionally, the study is post-hoc, as if we were attempting to trace the path the virus took three states earlier.
By analyzing genetically identical tissues from the initial stage of infection, one can reduce the noise of diversity and shed information on the course the virus travels. Which cells are infected, and when? What is the level of infection, and how does it differ depending on cell type? How does it change in different conditions?
And what if it were possible to track thousands of these infections at once? It might fundamentally alter how we think about infections and the medications we use to treat them.
That’s the hope for new advanced tech capable of growing mini organs on microchips. The labs of Rockefeller’s Ali Brivanlou and Charles M. Rice collaborated to refine a cell culture technology platform that grows genetically identical lung buds the embryonic structures that give rise to our breathing organs from human embryonic stem cells (hESCs). Their findings were recently published in Stem Cell Reports.
The hESCs quickly organize themselves into “micro lungs” with complete tissue complexity when placed on a grid of microchips and carefully dosed with a special cocktail of signaling chemicals. These buds can be cultivated in large numbers, enabling a hitherto unheard-of high-throughput examination of lung tissue infection free of distracting variables.
The end result is infinite, quick, and scalable access to human lung development-specific lung tissue that may be used to follow lung infections and find potential therapies.
“These lungs are basically clones,” says Ali Brivanlou. “They have the exact same DNA signature. That way we don’t have to worry about one patient responding differently from another. Quantification allows us to keep the genetic information constant and measure the key variable the virus.”
The platform will also allow us to respond to the next pandemic with much more speed and precision. We can quickly capitalize on this platform to make a virus visible and develop therapies much faster than we did for COVID. It can be used to screen for drugs, compounds, vaccines, monoclonal antibodies, and more directly in human tissue. This technology is ready to confront all kinds of threats that may hit us in the future.
Ali Brivanlou
Building a better mini lung
Embryonic stem cells are the Ur-cells of the human body. They can infinitely divide to create more stem cells or to differentiate into any other tissue. Brivanlou’s Laboratory of Synthetic Biology has long explored their potential.
Brivanlou joined forces with Rockefeller colleague Charles M. Rice during the COVID pandemic: his lab had the microchip technology to grow lung buds, and Rice’s lab had the necessary biosafety clearance required to infect them with SARS-Co-V2 and study the outcome.
In 2021, first authors Edwin Rosado-Olivieri, a stem cell biologist in Brivanlou’s lab, and Brandon Razooky, then a postdoc in Rice’s Laboratory of Virology and Infectious Disease, began coaxing the cells to organize into more specialized forms.
Stem cells don’t just organize on their own. They need a confined space such as a microchip well and stimuli to spark change. The stimuli that cause stem cells to develop into particular cell types come from four basic signaling pathways.
After roughly two weeks, the group’s lung cells had developed similar buds with molecular profiles that closely resembled those found in the initial stages of fetal lung development, including the production of airways and alveoli, structures that are known to be harmed in many persons with severe COVID.
Identifying a key culprit
Since then, they’ve used the platform to understand how SARS-Co-V2 infects different lung cells.
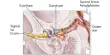
The tiny sacs known as alveoli are located at the tips of the lung branches and control the gas exchange that occurs with each breath: oxygen in, carbon dioxide out. The researchers found that alveoli, which are the guardians of the organ and the first line of defense against all inhaled threats, were more vulnerable to SARS-Co-V-2 infection than airway cells. If the virus got past them, the alveoli were sitting ducks.
Another view of virus particles (blue) infecting alveolar and airway tissues (red).
They also hit upon a winning combination of signaling proteins for creating the most robust batches of lung buds a mix of keratinocyte growth factor (KGF) and bone morphogenetic protein 4 (BMP4). Both contribute to cell differentiation and growth.
Interestingly, the BMP pathway has a downside. They discovered that the BMP signaling pathway was activated in both infected lung buds and postmortem tissue from COVID patients, making the tissues more susceptible to infection. Blocking the BMP pathway made the cells less vulnerable.
Beyond COVID
The platform can also be used to look at the causes of diseases including lung cancer, RSV, pulmonary illnesses, and influenza, according to the experts. Moreover, it can be used to screen for new drugs to treat them.
And lungs are far from the only organ of interest. “The broader focus of our work is understanding cellular development to make synthetic organs and tissues that we can use to model diseases and find therapeutic mechanisms,” says Rosado-Olivieri. The liver, kidney, and pancreas are all likely next targets.
“The platform will also allow us to respond to the next pandemic with much more speed and precision,” Brivanlou adds. “We can quickly capitalize on this platform to make a virus visible and develop therapies much faster than we did for COVID. It can be used to screen for drugs, compounds, vaccines, monoclonal antibodies, and more directly in human tissue. This technology is ready to confront all kinds of threats that may hit us in the future.”