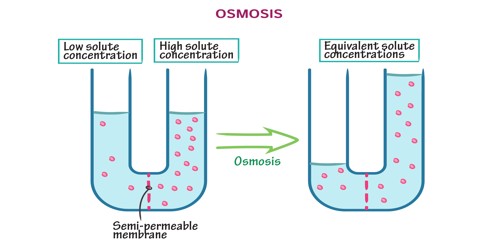
Osmosis is the movement of water or other solvents through a plasma membrane from a region of low solute concentration to a region of high solute concentration, tending to equalize the concentrations of the solutes. It is the spontaneous passage or diffusion of water or other solvents through a semipermeable membrane. It is a special type of diffusion, namely the diffusion of water across a semipermeable membrane. Osmosis is passive transport, meaning it does not require energy to be applied. It is a biophysical phenomenon occurring commonly in biologic systems, in which cells of fluid compartments are separated by semipermeable membranes. What causes osmotic pressure is different concentrations of solutes on the two sides of the membrane. It describes the diffusion of the solvent through a semipermeable membrane. It happens spontaneously and without any energy on the part of the cell. When molecules move in and out of a cell to achieve the same concentration of something, like salt, on both sides, then osmosis is happening. Any solvent can undergo the process of osmosis including gases and supercritical liquids.
Osmosis is the process in plants and animals by which a liquid moves gradually from one part of the body or the plant to another through a membrane. This process can be stopped by increasing the pressure on the solution by a specific amount, called the osmotic pressure. It is the force required to prevent water movement across the semipermeable membrane. Osmosis affects plant and animal cells differently because plant and animal cells can tolerate different concentrations of water. It can have adverse effects on animals such as fish.
When osmosis happens, water moves from the side of the membrane with a lower amount of osmotic pressure to the side of the membrane with the higher amount. It is the result of diffusion across a semipermeable membrane from a lower concentration to a higher one. An important example of osmosis is the movement of liquid (solvent) molecules across a cell membrane into a cell with a higher solute concentration. Osmosis is a fundamental part of cell biochemistry but also has mechanical applications and usages.
Importance
- Osmosis influences the transport of nutrients and the release of metabolic waste products.
- It is responsible for the absorption of water from the soil and conducting it to the upper parts of the plant through the xylem.
- It maintains the turgidity of cells.
- This process controls the cell to cell diffusion of water.
- Osmosis induces cell turgor which regulates the movement of plants and plant parts.