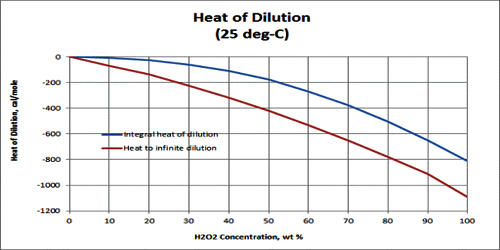
The heat of dilution refers to the enthalpy change associated with the dilution process of a component in a solution at a constant pressure. It is the heat evolved per mole of a solute when a solution is greatly diluted. It is the increase in enthalpy accompanying the addition of a specified amount of solvent to a solution of constant pressure. Usually, the enthalpy of dilution of a component in a solution is expressed in terms of energy per amount of substance.
If the initial state of the component is a pure liquid (presuming the solution is liquid), the dilution process is equal to its dissolution process and the heat of dilution is the same as the heat of solution. In principle, the overall enthalpy of dilution can be considered to include one term due to the solute and another one due to the solvent. During a solution process, a solute is transferred from a pure solute phase (solid, liquid, or gas) to a solvent or solution phase. Generally, the heat of dilution is normalized by the mole number of the solution and its dimensional units are energy per unit mass or amount of substance, commonly expressed in the unit of kJ/mol (or J/mol). During a dilution process, the solvent is transferred from a pure solvent phase to a solution phase. However, this quantity can also be expressed in terms of energy per unit mass. The most common units used to express enthalpy of dilution are joules per mole (J/mol) and kilojoules per mole (kJ/mol).
Given that a solution exists in the liquid phase if a pure liquid component is dissolved into the solution, the enthalpy of dilution will be the same as the enthalpy of dissolution. This is because the dilution process in this scenario is the same as the dissolution process for the component being dissolved. Enthalpy of Reaction (Heat of Reaction) is the heat liberated or the heat absorbed when a chemical reaction takes place.
- An exothermic reaction liberates heat, the temperature of the reaction mixture increases.
- An endothermic reaction absorbs heat, the temperature of the reaction mixture decreases.