An alicyclic compound is an organic compound that is both aliphatic and cyclic. It refers to aliphatic compounds in which some of the carbon atoms are in a ring formation, but may not be a benzenoid or other aromatic system. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. The name alicyclic is derived from the combination of “ali” from aliphatic and “cyclic” to indicate that it is an enclosed structure. Alicyclic compounds may have one or more aliphatic side chains attached. These alicyclic compounds can be either saturated or unsaturated, but they are not aromatic. Saturated means that there are no double or triple bonds between atoms; unsaturated means the opposite of it.
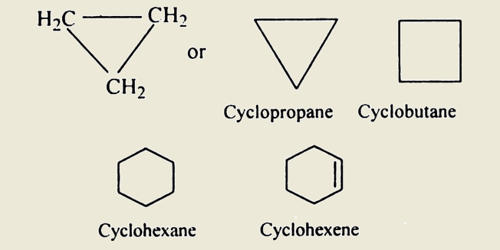
The simplest alicyclic compounds are the monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, cyclooctane, and so on. Bicyclic alkanes include bicycloundecane, decalin, and housane. Polycyclic alkanes include cubane, basketane, and tetrahedrane. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character.
Alicyclic compounds may have one or more aliphatic side chains attached. Spiro compounds have two or more rings that are connected through only one carbon atom. Those alicyclic compounds in which the ring contains three or four carbon atoms are less stable than the compounds having larger rings because the angles formed by adjacent covalent bonds are smaller than is necessary for maximum effectiveness. The mode of ring-closing in the formation of many alicyclic compounds can be predicted by Baldwin’s rules.
Otto Wallach, a German chemist, received the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds.
Characteristics –
- The stability of alicyclic compounds depends on the chemical structure of the compound.
- They do not have delocalized pi-electron clouds.
- They can be either saturated or unsaturated compounds.
- They have no specific aroma.
- They are compounds that contain one or more aromatic rings.
- Their reactivity is similar to that of their open-chain alkane counterparts.