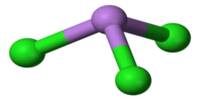
Gadolinium oxychloride (GdOCl) is an inorganic compound composed of gadolinium, oxygen, and chlorine. It typically appears as a crystalline solid, often white or pale in color, and belongs to the family of rare-earth oxyhalides. It can be synthesized through controlled reactions involving gadolinium oxide or gadolinium chloride under suitable conditions.
Structurally, gadolinium oxychloride exhibits a layered arrangement where oxygen and chlorine atoms coordinate with gadolinium ions. This crystalline arrangement is significant because it influences the compound’s optical, electronic, and magnetic properties. Being a gadolinium-based material, it shows paramagnetism, a property that makes gadolinium and its derivatives valuable in certain magnetic and imaging applications.
Properties
Gadolinium oxychloride forms crystals of the tetragonal system, space group P4/nmm. The compound is insoluble in water but dissolves slowly in hydrochloric and nitric acids.
- Chemical formula: GdOCl
- Molar mass: 208.70 g/mol
- Appearance: crystals
- Solubility in water: insoluble
- Crystal structure: Layered oxyhalide structure, where gadolinium ions are coordinated by both oxygen and chlorine.
- Density: Relatively high, due to the presence of the heavy rare earth element gadolinium.
- Magnetic properties: Gadolinium compounds generally exhibit paramagnetism or even ferromagnetic behavior at low temperatures.
- Thermal stability: Stable at moderate temperatures, but decomposes to gadolinium oxide (Gd₂O₃) and gadolinium chloride (GdCl₃) upon strong heating.
- Solubility: Slightly soluble in water; hydrolyzes gradually, releasing chloride ions and forming hydrated oxides.
- Reactivity: Can react with moisture, acids, or bases, leading to further hydrolysis or salt formation.
Occurrences
- Natural occurrence: Gadolinium oxychloride does not occur naturally; it is a synthetic compound. Gadolinium itself is found in rare earth minerals such as monazite and bastnäsite.
- Synthesis: Prepared by controlled hydrolysis of gadolinium chloride (GdCl₃) or by direct reaction between gadolinium oxide (Gd₂O₃) and gadolinium chloride at elevated temperatures.
Applications
One of its most important uses is as a host material in phosphors. Doped with activator ions such as europium or terbium, gadolinium oxychloride can emit strong luminescence, making it useful in x-ray intensifying screens, fluorescent lamps, and display technologies. Its good stability, relatively high density, and efficient light conversion enhance its application in medical imaging, particularly for improving radiographic sensitivity.