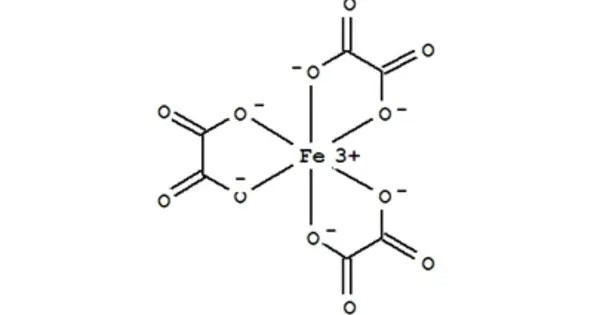
Ferric oxalate, also known as iron(III) oxalate, refers to inorganic compounds with the formula Fe2(C2O4)3(H2O)x but could also refer to salts of [Fe(C2O4)3]3-. It is usually a yellow-brown or brown powder. It is sparingly soluble in water but dissolves more readily in acids.
Fe2(C2O4)3(H2O)x are coordination polymers with varying degrees of hydration. The coordination complex with the formula [Fe(C2O4)3]3- forms a variety of salts, a well-known example being potassium ferrioxalate. It is often used in various chemical processes, especially in analytical chemistry and photochemistry.
Properties
Ferric oxalate typically appears as a yellow-brown or light-brown solid, and it can be in a powder or crystalline form. It is relatively insoluble in water but may dissolve in acidic solutions due to the presence of the oxalate ion. The solubility increases when heated in the presence of acids. It is relatively stable under normal conditions, but it can decompose at higher temperatures or under reducing conditions.
- Chemical formula: C6Fe2O12
- Molar mass: 375.747 g/mol
- Appearance: Pale yellow solid (anhydrous), Lime green solid (hexahydrate)
- Odor: odorless
- Melting point: 365.1 °C (689.2 °F)
- Solubility in water: slightly soluble
Production
Ferric oxalate may be produced by reaction of iron(III) hydroxide and solution of oxalic acid:
2Fe(OH)3 + 3H2C2O4 → Fe2(C2O4)3 + 6H2O
Natural Occurrence
Ferric oxalate can be found in nature, though it is relatively rare. It may occur in soils and plants in small amounts, as oxalates are common in plants. In some cases, ferric oxalate can also be found as part of the mineral structure of certain rocks or as a byproduct of iron-rich mineral weathering.
Formation in Plants
Many plants, particularly those in the Oxalis genus (like wood sorrels), naturally produce oxalates, which may combine with iron to form ferric oxalate. In such plants, ferric oxalate can accumulate, although it is typically found in trace amounts.
Synthetic Occurrence
Ferric oxalate is most commonly produced in the laboratory or industrial processes. It can be synthesized by reacting iron(III) salts, such as iron(III) chloride (FeCl₃) or iron(III) nitrate (Fe(NO₃)₃), with oxalic acid (H₂C₂O₄) under controlled conditions. This process is often carried out in aqueous solutions to yield ferric oxalate in its hydrated form.
Uses
- Dentistry – Like many oxalates, ferric oxalate has been investigated as a short-term treatment for dentin hypersensitivity. It is used in certain toothpaste formulations; however, its effectiveness has been questioned.
- Photography – It is used as the light-sensitive element in the Kallitype photographic printing process; and the platinotype process Platinum/Palladium Printing.
- Batteries – It has been investigated as a possible cheap material for the positive electrode of lithium-iron batteries. It can intercalate lithium ions at an average potential of 3.35 V, and has shown a sustainable capacity of 98 mAh/g.
Toxicity
Ferric oxalate, like other oxalates, can be toxic if ingested. The oxalate ion can bind to calcium in the body, forming insoluble calcium oxalate crystals, which could lead to kidney stones or other health issues in excessive amounts. Thus, proper handling is required in laboratory settings.