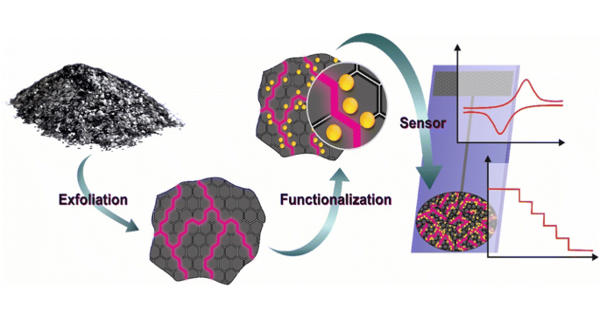
Nanomaterials are frequently dispersed in solvents in industrial applications in order to be embedded in final products. Battery electrodes, composite reinforcement, printed electronics, and many other applications are examples. Because nanomaterials have a high surface-to-mass ratio, their surface properties, specifically surface chemistry, govern their interactions with the outside world. As a result, in order to optimize product performance, these must be carefully characterized.
Characterizing nanomaterial dispersions, on the other hand, is difficult due to the small length scales involved, as well as the use of liquids, which are incompatible with the vacuum conditions used in many ex-situ techniques. These traditional measurement methods can be expensive and time consuming, and the pre-processing steps commonly used to remove nanomaterials from liquid can potentially alter their material properties.
Characterizing nanomaterial dispersions is difficult to achieve because of the small length scales, as well as the use of liquids, which are incompatible with the vacuum conditions employed in many ex-situ techniques.
Elemental 2D materials have emerged as promising candidates for electrochemical applications requiring miniaturized devices and high performance. These atomically thin materials are primarily derived from bulk-layered materials with strong in-plane covalent bonding and weak interlayer van der Waals bonding. Their large surface areas, high structural variability, and electronic properties make them clearly superior for energy storage systems (ESSs). This review describes the properties and electrochemical applications of elemental 2D nanomaterials such as gas sensing, catalysis, and ESS.
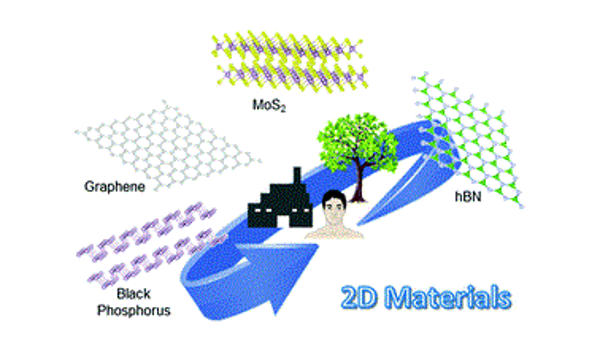
Two-dimensional carbon materials are very appealing for use in a wide range of sensing applications. Graphene is investigated as a recognition layer in electrochemical sensors such as field-effect transistors, chemiresistors, impedance-based devices, and voltammetric and amperometric sensors in this paper. The sensor performance is evaluated from a material standpoint, revealing the impact of structure and defects in 2D carbon materials in various transducing technologies. It is concluded that the performance of 2D carbon-based sensors is highly dependent on the preparation method in conjunction with the electrical transduction technique.
Chemical synthesis of 2D materials allows for a great deal of flexibility in process parameters. So far, these possibilities have not been fully realized, for example, by designing 2D materials with a controlled number of defects. Such materials could be very useful in sensing applications. The structural irregularities act as nucleation sites for the particles during surface functionalization by nanoparticle (NP) deposition. Defects enable the introduction of bandgaps, which may be advantageous in field-effect transistors.
Researchers from NPL’s Surface Technology group demonstrated the use of nuclear magnetic resonance (NMR) proton relaxation to rapidly characterize the surface chemistry of 2D graphitic particles in liquids in several minutes and without the need for any sample preparation in a study featured on the front cover of Nanoscale. Because NMR relaxation can be performed in-line, it has the potential to be used as a quality control method by manufacturers of nanomaterials.