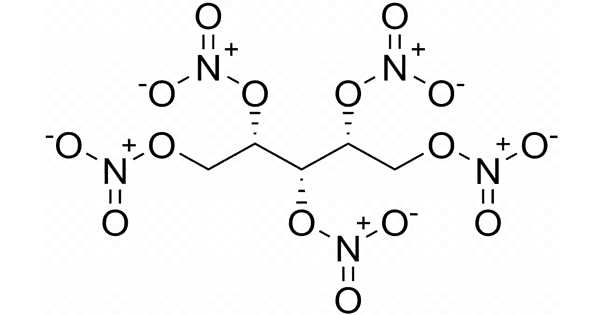
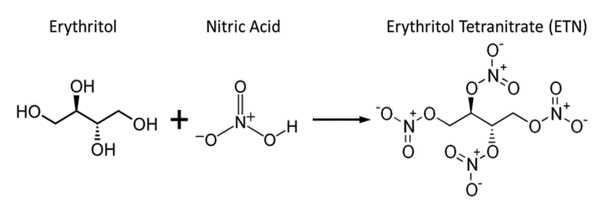
Erythritol tetranitrate (ETN) is a chemically similar explosive compound to PETN, though it is thought to be slightly more sensitive to friction and impact. Each of the hydroxy groups in erythritol has been converted to the corresponding nitrate ester. It is a vasodilator with similar properties to nitroglycerin.
ETN, like many nitrate esters, acts as a vasodilator and was the active ingredient in the first “sustained release” tablets, manufactured under a process patent called “nitroglyn” in the early 1950s. Ingestion of ETN or prolonged skin contact can result in absorption and a “nitro headache.”
Properties
At a density of 1.7219 (0.0025) g/cm3, ETN has a relatively high detonation velocity of 8206 m/s. It is odorless and white in color. ETN is frequently mixed with other high explosives. Because it is sensitive to shock and friction, it must be handled with caution. ETN is easily dissolved in acetone and other ketone solvents. The sensitivity to impact and friction is slightly higher than that of pentaerythritol tetranitrate (PETN). Melt cast and pressed ETN to have comparable sensitivity. Lower erythritol nitrates, such as erythritol trinitrate, are water-soluble and thus do not contaminate most ETN samples.
ETN, like PETN, is known for having a long shelf life. After four years of storage at room temperature, studies that directly observed the crystalline structure found no signs of decomposition. PETN has a melting point of 141.3°C, while ETN has a melting point of 61°C. Recent ETN decomposition studies have suggested a unimolecular rate-limiting step in which the O-NO2 bond is cleaved and the decomposition sequence begins.
Oxygen balance
This explosive has a positive oxygen balance, which means it has more than enough oxygen in its structure to fully oxidize all of its carbon and hydrogen upon detonation, which PETN does not. This is depicted in the chemical equation schematic below.
2 C4H6N4O12 → 8 CO2 + 6 H2O + 4 N2 + 1 O2
Whereas PETN decomposes to:
2 C5H8N4O12 → 6 CO2 + 8 H2O + 4 N2 + 4 CO
Carbon monoxide (CO) still needs oxygen to be completely oxidized to carbon dioxide (CO2). A thorough examination of the chemistry of ETN decomposition has recently been completed.
As a result, for every two moles of ETN decomposed, one mole of free O2 is released. This oxygen could be used to oxidize additional metal dust or an oxygen-deficient explosive like TNT or PETN. The following chemical equation depicts how oxygen from ETN oxidizes PETN. Carbon monoxide (CO) is converted to carbon dioxide by the extra oxygen provided by the ETN (CO2).
2 C4H6N4O12 + 1 C5H8N4O12 → 13 CO2 + 10 H2O + 6 N2
Manufacture and Uses
ETN, like other nitrated polyols, is produced by nitrating erythritol with either concentrated sulfuric acid and a nitrate salt or a mixture of sulfuric and nitric acid.
To reduce the risk of explosion, it is usually diluted with lactose or other suitable inert excipients; undiluted erythrityl tetranitrate can be exploded by percussion or excessive heat. It functions as both a vasodilator and an explosive. It is derived from erythritol.
Information Source: