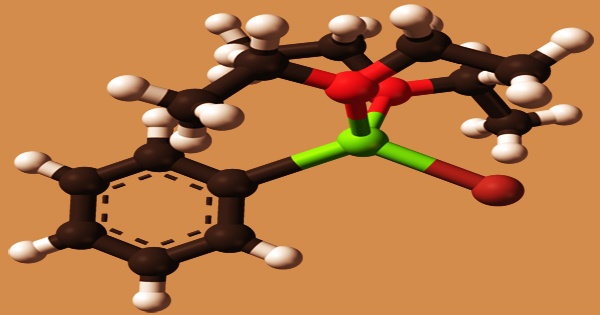
The aryl magnesium halide phenylmagnesium bromide, having the simplified formula C6H5MgBr, serves as a Grignard reagent. It’s an organometallic chemical that contains magnesium. It’s sold as a solution in either diethyl ether or tetrahydrofuran (THF). L.Mond discovered in 1890 that burning carbon monoxide after passing it through active metal nickel powder produced a green light flame.
After cooling the resultant gas, he obtained a colorless liquid (melting point 298K, boiling point 316K); when this gas is passed through a heated glass tube, the metallic nickel deposits on the wall may be observed. A Grignard reagent is phenylmagnesium bromide. It is frequently used as a substitute for the phenyl “Ph−“ synthon. Grignard reagents are an organomagnesium halide with the formula RMgX, where X is a halogen and R is an alkyl or aryl (benzene ring-based) group.
Bromobenzene is treated in the lab with magnesium metal, which is generally in the form of turnings. To start the process, a tiny quantity of iodine can be used to activate the magnesium. The Grignard reagent is added to a ketone or aldehyde to create tertiary or secondary alcohol, respectively, in the Grignard reaction. Since the 1960s, hundreds of different carbonyl compounds and derivatives have been created.
Almost every transition metal has the ability to create such compounds. Metal carbonyls, also known as carbonyl complexes, are a type of unusual complex produced by transition metals and carbon monoxide ligands. To solvate (complex) the magnesium(II) core, coordination solvents such as ether or THF are required.
Because alcohols and water contain an acidic proton, they react with phenylmagnesium bromide to produce benzene, the solvent must be aprotic. The reagent is also incompatible with carbonyl-containing solvents like acetone and ethyl acetate. A primary alcohol is formed when formaldehyde reacts with it. The Grignard reagent may be used to figure out how many halogen atoms are in a halogen compound.
In contemporary inorganic chemistry, metal carbonyl plays a major role in both theoretical and practical studies. The material’s influence on air quality, possible migration in air, soil, or water, impacts on an animal, aquatic, and plant life, and compliance with environmental and public health standards must all be considered in the final disposal of the chemical. The majority of metal carbonyl complexes are made by combining metal and carbon monoxide directly.
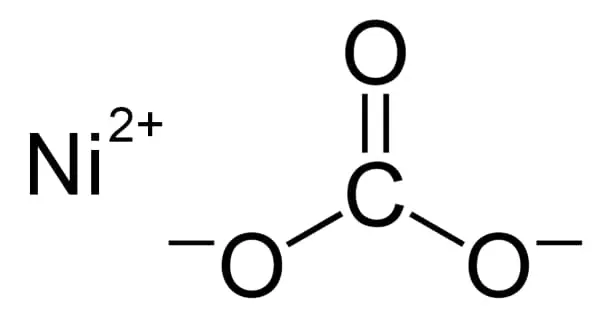
The metal, on the other hand, must be brand new reduction products in a highly active condition. Despite the fact that phenylmagnesium bromide is commonly written as C6H5MgBr, the molecule is more complicated. With two OR2 ligands from the ether or THF solvent, the molecule usually forms an adduct. As a result, the magnesium is tetrahedral and follows the octet rule.
The Mg–O lengths are 201 and 206 pm, respectively, whereas the Mg–C, and Mg–Br distances are 220 and 244 pm. Carbonylation reduction under high pressure. Under high pressure, apply a reducing agent to enable the carbonylation reaction between the metal and the carbonyl group, with the most common reducing agents being hydrogen, active metals, phenylmagnesium bromide (C6H5MgBr), and others.
Phenylmagnesium bromide is both a powerful nucleophile and a powerful base. Certain polynuclear carbonyl compounds can be obtained by thermal or UV irradiation breakdown. Heteronuclear carbonyl compounds are formed when two distinct types of metal carbonyl compounds interact with one other. Because it may absorb even moderately acidic protons, the substrate must be safeguarded where possible. It frequently reacts with carbonyls like ketones and aldehydes.
The coupling product is formed when phenylmagnesium bromide reacts with ferric chloride. Ferrocene is formed when cyclopentadienyl magnesium bromide reacts with ferric chloride. Hexaphenyldilead is an antioxidant for polyphenylene ether lubricants, which are made by reacting lead chloride with phenyl lithium or phenyl magnesium bromide. After an acidic workup, it interacts with carbon dioxide to produce benzoic acid. Triphenylphosphine can be produced by reacting three equivalents with phosphorus trichloride.
The potassium ion, ammonium ion, rubidium ion, and cesium ion are all determined using sodium tetraphenylborate. It is made by reacting phenylmagnesium bromide with boron trifluoride/diethyl ether to produce Tetraphenylboron magnesium bromide. To obtain it, evaporate the diethyl ether, add water, and then treat it with the specified amount of sodium carbonate.
Information Sources: