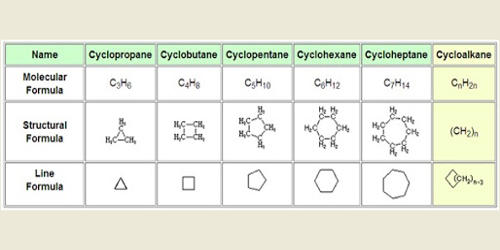
In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. They consist of carbon and hydrogen atoms that are saturated because of the single carbon-carbon bond. They are cyclic hydrocarbons, meaning that the carbons of the molecule are arranged in the form of a ring. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. They are also saturated, meaning that all of the carbons atoms that make up the ring are single bonded to other atoms.
Cycloalkanes are types of alkanes that have one or more rings of carbon atoms in their structure. They are named analogously to their normal alkane counterparts of the same carbon count: cyclopropane, cyclobutane, cyclopentane, cyclohexane, etc. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. There are also polycyclic alkanes, which are molecules that contain two or more cycloalkanes that are joined, forming multiple rings. The cycloalkanes without side chains are classified as small (cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium (cyclooctane through cyclotridecane), and large (all the rest). The ring structure prevents cycloalkanes from achieving a tetrahedral configuration around their carbon atoms, which produces ring strain.
The physical properties of cycloalkanes are similar to those of alkanes, but they have higher boiling points, melting points, and higher densities due to the greater number of London forces that they contain. Besides this standard definition by the International Union of Pure and Applied Chemistry (IUPAC), in some authors’ usage, the term cycloalkane includes also those saturated hydrocarbons that are polycyclic. They are named similarly to their linear counterparts, but with the prefix “cyclo-,” such that a three-carbon ring, for example, would be cyclopropane. In any case, the general form of the chemical formula for cycloalkanes is CnH2(n+1−r), where n is the number of carbon atoms and r is the number of rings. The simpler form, when working without focusing on rings is CnH2(n).
Cycloalkanes are named using the same conventions as linear alkanes, with the prefix cyclo- added to the front of the name. Generally, the melting point, the boiling point, and the density of cycloalkanes increase as the number of carbons increases. This trend occurs because of the greater number of bonds that are in higher membered rings, thus making the bonds harder to break. Many cycloalkanes are used in motor fuel, natural gas, petroleum gas, kerosene, diesel, and many other heavy oils.