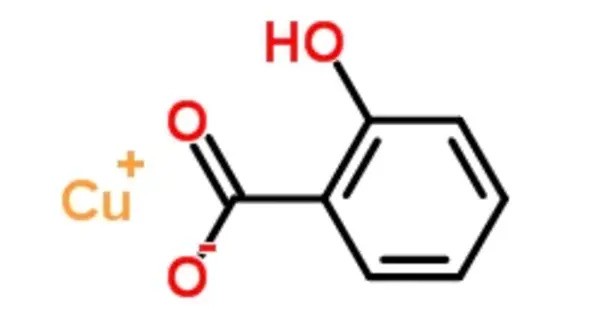
Copper salicylate describes a range of compounds containing copper(II) and salicylate. It is a coordination compound formed from copper and salicylic acid, a type of aromatic carboxylic acid with known anti-inflammatory properties. This compound is of interest in both pharmaceutical and materials science fields due to its potential biological activity and unique chemical structure.
As a metal-organic complex, Copper Salicylate exhibits properties distinct from its individual components, including enhanced antimicrobial, anti-inflammatory, and possibly antioxidant effects. It is also studied for its thermal stability, coordination chemistry, and potential applications in medicinal and catalytic contexts. Many compounds are known.
They are generally blue. Simple species include:
Cu(O2C6H4OH)2(H2O)2·H2O.
Cu2(2O2C6H4OH)4(H2O)2·L) (L = diverse solvent.
Many adducts of copper(II) salicylates are known with amines and N-hetrerocyclic ligands.
Chemical Structure and Composition
- Formula: Typically represented as 𝐶𝑢(𝐶7𝐻5𝑂3)2·xH₂O (where C₇H₅O₃⁻ is the salicylate anion).
- Coordination: The copper(II) ion (Cu²⁺) is usually coordinated by two salicylate anions, which can bind via the phenolic hydroxyl and carboxylate groups, creating a chelate ring.
- Color: Dark green or bluish-green crystalline solid.
- Solubility: Slightly soluble in water; more soluble in alcohols and organic solvents depending on hydration and synthesis method.
Property
- Molecular Weight: Depends on hydration level; approx. 369.92 g/mol (anhydrous form)
- Melting Point: Decomposes before melting
- Magnetism: Paramagnetic due to the presence of unpaired electrons in Cu²⁺
- Stability: Stable under normal conditions; decomposes at high temperatures
- Solubility in solvents: Slight in water, better in ethanol, acetone, and DMSO
Uses
- Medical research: Investigated for anti-inflammatory and anticancer properties.
- Antifungal/antimicrobial: Copper ions enhance antimicrobial activity.
- Analytical chemistry: Used in complexometric and spectrophotometric studies.
- Coordination chemistry: Studied for ligand behavior and transition metal interactions.