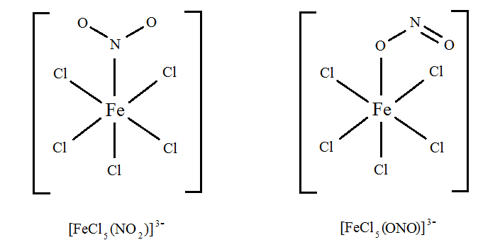
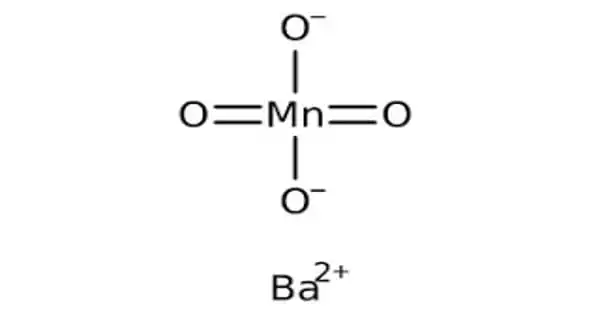
Coordination isomerism is a form of structural isomerism in which the composition of the complex-ion varies. It is a form of structural isomerism in which the composition of the complex-ions varies. It occurs compounds containing complex anionic and cationic parts that can be thought of as occurring by an interchange of some ligands from the cationic part to the anionic part. In a coordination isomer, the total ratio of ligand to metal remains the same, but the ligands attached to a specific metal ion change.
In coordination isomerism, both positive and negative ions of salt are complex-ions and the two isomers differ in the distribution of ligands between the cation and the anion. Compounds having the same molecular formula but different structures or spatial arrangements are called isomers and the phenomenon is referred to as isomerism. Coordination isomerism refers to cases where there are different ways to arrange several ligands around two metal centers. For example, there are several ways to arrange six CN− ions and six NH3 molecules around two metal ions that total +6 in the oxidation number. This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex.
Coordination isomers are coordination salts in which there is an interchange of ligands between the metal in the cation and the metal in the anion. In a coordination isomer, the total ratio of ligand to metal remains the same, but the ligands attached to a specific metal ion change. Due to their complicated formulae of many coordination compounds, the variety of bond types, and the number of shapes possible, many different types of isomerism occur. They are two or more coordination compounds in which the composition within the coordination sphere (i.e., the metal atom plus the ligands that are bonded to it) is different (i.e., the connectivity between atoms is different). Examples of a complete series of coordination isomers require at least two metal ions and sometimes more.
Coordination isomers have different physical and chemical properties. For example, a solution containing ([Co(NH3)6]3+ and [Cr(CN)6]3−) is a coordination isomer with a solution containing [Cr(NH3)6]3+ and [Co(CN)6]3−.