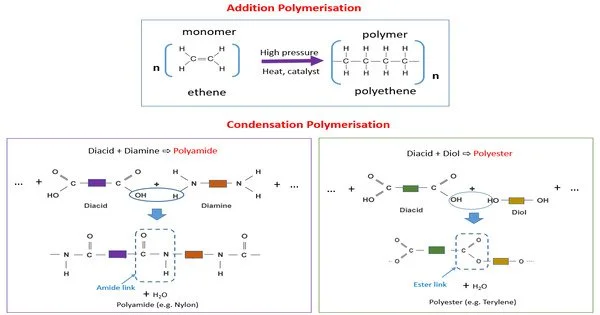
Condensation polymers are any polymers whose polymerization process involves a condensation reaction (i.e., a tiny molecule, such as water or methanol, is created as a byproduct). Step-growth polymers are a type of polymer generated by a chemical reaction in which monomers mix, resulting in the elimination of a tiny molecule (typically water or another simple substance) as a byproduct. This approach differs from addition polymers in that monomers are simply added together with no byproducts eliminated.
Polycondensation polymers are created by condensation reactions between species of all degrees of polymerization, or by condensative chain polymerization, which is formed by consecutive addition of monomers to an active site in a chain reaction. Chain polymerization and polyaddition are the two basic types of polymerization that produce addition polymers.
Step-growth polymerization is a type of condensation polymerization. Linear polymers are made from bifunctional monomers, which have two reactive end groups. Polyamides, polyacetals, and proteins are examples of condensation polymers.
Here are the key characteristics and steps involved in the formation of condensation polymers:
- Monomers: These are formed from bifunctional or polyfunctional monomers. These monomers have two or more reactive functional groups, such as -OH (hydroxyl), -COOH (carboxyl), -NH2 (amine), or -SH (sulfhydryl). Examples of common monomers for condensation polymers include dicarboxylic acids and diamines or diols.
- Condensation Reaction: The polymerization process involves a condensation reaction between the functional groups of the monomers. During this reaction, one functional group from one monomer and another functional group from another monomer combine to form a covalent bond. Simultaneously, a small molecule, typically water (H2O), is eliminated as a byproduct.
- Repeating Units: As the condensation reaction progresses, it creates a chain of repeating units connected by covalent bonds. The polymer chain is made up of these repeating units.
- Molecular Weight: In contrast to addition polymers (such as polyethylene or polypropylene), which have a relatively limited molecular weight distribution, condensation polymers frequently have a wider range of molecular weights. This is due to the fact that the reaction might halt at various phases, resulting in polymers of varying lengths.
- Byproducts: Condensation polymerization is distinguished by the removal of tiny molecules (often water) as byproducts. To drive the process toward higher molecular weight polymers, these byproducts must be eliminated or collected.
Examples: Some common examples of condensation polymers include:
- Polyesters: Formed from the condensation of diacids (e.g., terephthalic acid) and diols (e.g., ethylene glycol).
- Polyamides (Nylons): Created by condensing diacids with diamines (e.g., adipic acid and hexamethylene diamine).
- Polycarbonates: Produced from the condensation of a diol (e.g., bisphenol A) and a carbonyl chloride (e.g., phosgene).
Condensation polymers frequently have strong thermal and mechanical properties, making them useful in a variety of applications such as textiles, plastics, and engineering materials. Condensation polymers are very versatile in terms of tailoring attributes to match specific requirements due to their capacity to incorporate a wide range of functional groups into their structures.