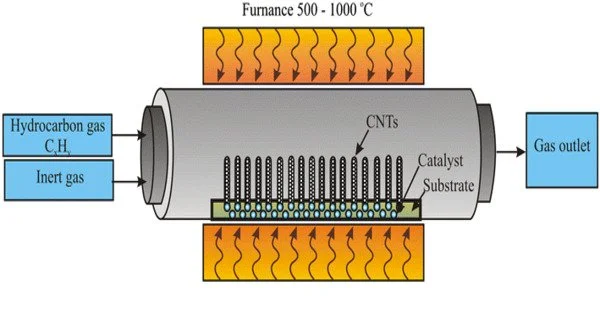
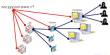
Chemical vapor deposition (CVD) is a process used to deposit thin films of various materials onto a substrate. It involves the reaction of one or more volatile precursors, usually in the gas phase, that are brought into contact with a heated substrate. The precursors react and deposit onto the substrate, forming a thin film.
CVD is a vacuum deposition method that is used to create high-quality, high-performance solid materials. Thin films are frequently produced using this method in the semiconductor industry. Typical CVD involves exposing the wafer (substrate) to one or more volatile precursors, which react and/or decompose on the substrate surface to form the desired deposit. Volatile by-products are frequently produced and removed by gas flow through the reaction chamber.
CVD is widely used in microfabrication processes to deposit materials in a variety of forms, including monocrystalline, polycrystalline, amorphous, and epitaxial. Silicon (dioxide, carbide, nitride, oxynitride), carbon (fiber, nanofibers, nanotubes, diamond, and graphene), fluorocarbons, filaments, tungsten, titanium nitride, and various high-dielectrics are among these materials.
John M. Blocher, Jr. coined the term chemical vapour deposition in 1960 to distinguish it from physical vapour deposition (PVD).
The CVD process typically involves four main steps:
- Preparation: The substrate is cleaned and prepared before the CVD process. This may involve cleaning the substrate with solvents, plasma, or other techniques to remove any contaminants or impurities.
- Deposition: The precursors are introduced into the reaction chamber, where they react to form the desired material. The reaction typically takes place at high temperatures and under vacuum or reduced pressure.
- Growth: The deposited material grows on the substrate as the precursors continue to react and deposit onto the surface.
- Termination: The CVD process is stopped, and the deposited material is cooled down and removed from the reaction chamber.
CVD can be performed using a variety of different methods, including atmospheric pressure CVD (APCVD), low-pressure CVD (LPCVD), plasma-enhanced CVD (PECVD), and metal-organic CVD (MOCVD). Each method has its own advantages and disadvantages and is chosen based on the specific application and requirements of the thin film being deposited.
Uses
CVD is commonly used in the semiconductor industry for the production of thin films used in the manufacture of electronic components. It is also used in other industries for a variety of purposes, such as the deposition of coatings on optical lenses and the production of hard coatings for cutting tools.
CVD can also deposit conformal films and augment substrate surfaces in ways that other surface modification techniques cannot. In the process of atomic layer deposition, CVD is extremely useful for depositing extremely thin layers of material. Such films have a wide range of applications.
Some integrated circuits (ICs) and photovoltaic devices contain gallium arsenide. Photovoltaic devices make use of amorphous polysilicon. Wear resistance is conferred by certain carbides and nitrides. CVD polymerization, perhaps the most versatile of all applications, enables super-thin coatings with desirable properties such as lubricity, hydrophobicity, and weather resistance, to name a few. Recently, CVD of metal-organic frameworks, a class of crystalline nanoporous materials, was demonstrated.