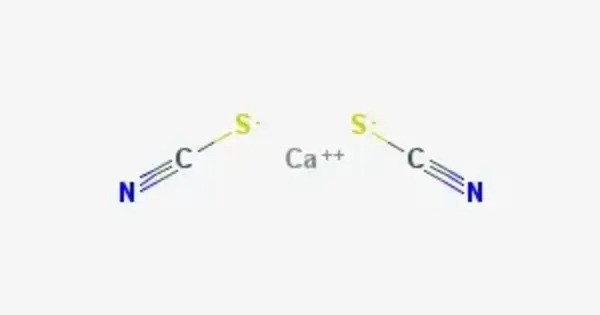
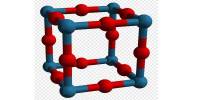
Calcium thiocyanate is an inorganic compound with the chemical formula Ca(SCN)₂, consisting of calcium cations (Ca²⁺) and thiocyanate anions (SCN⁻). It is a colorless solid. According to X-ray crystallography, it is a coordination polymer. The Ca2+ ions are each bonded to eight thiocyanate anions, with four Ca-S and four Ca-N bonds. The motif is reminiscent of the fluorite structure.
The compound is usually prepared by neutralizing thiocyanic acid (HSCN) with calcium hydroxide or calcium carbonate. It can also be obtained through double decomposition reactions involving calcium salts and alkali thiocyanates. One of its main uses is in the production of polymer and resin systems, where it functions as a stabilizer or additive. It also finds application in the textile and dyeing industry, particularly in creating complex dye formulations, as thiocyanates can act as ligands for transition metals.
Properties
It typically appears as a white to slightly yellow solid, soluble in water and alcohols. In aqueous solutions, it dissociates into ions, exhibiting properties similar to other soluble thiocyanates.
- Chemical formula: C2CaN2S2
- Molar mass: 156.23 g·mol−1
- Appearance: white solid
- Solubility: Highly soluble in water and soluble in alcohols. The aqueous solution often has a sharp odor due to hydrolysis and thiocyanate ion activity.
- Stability: Hygroscopic in nature; absorbs moisture from the air. It decomposes on strong heating, releasing toxic gases such as carbon disulfide, sulfur oxides, and nitrogen oxides.
Occurrences
- Calcium thiocyanate is synthetic and does not occur naturally in mineral form. It is generally produced by neutralizing thiocyanic acid (HSCN) with calcium hydroxide or calcium carbonate.
- Industrially, it is used in agriculture as a component in certain fungicides, herbicides, and soil treatment agents, due to its pesticidal properties.
- It has applications in the textile industry (as a chemical intermediate), and in the production of thiourea.
- Occasionally, it is used in analytical chemistry as a source of SCN⁻ ions to test for ferric ions.