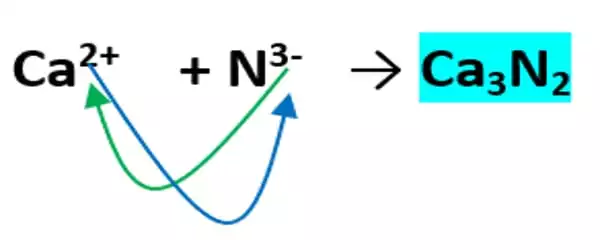
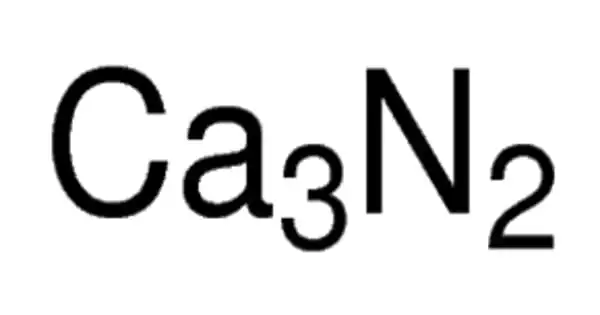Calcium nitride is the inorganic compound with the chemical formula Ca3N. It exists in various forms (isomorphs), α-calcium nitride being more commonly encountered. It has different isomorphous forms, out of which α-calcium nitride is commonly found. It is made up of calcium and nitrogen. It can be used as a source of reactive nitride ion.
Calcium nitride has been used as a high-temperature solvent in the recrystallization of refractory aluminum nitride, which is the process of growing single crystals of aluminum nitride from a molten solution containing the refractory material as the solute and calcium nitride as the solvent. The precipitation or crystallization of the solute, the solute is the desired crystal, from the molten solution results in crystallization.
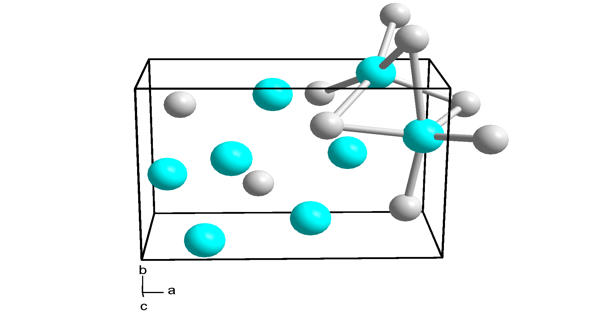
Structure
α- Calcium nitride has an anti-bixbyite structure, similar to Mn2O3, except the ion positions are reversed: calcium (Ca2+) takes the oxide (O2) positions, and nitride ions (N3) takes the manganese (Mn3+). Ca2+ occupies tetrahedral sites in this structure, while nitride centers occupy two different types of octahedral sites.
Properties
It is a red-brown solid. It reacts with water to make calcium hydroxide and ammonia. It reacts with hydrogen to make calcium hydride and calcium amide. It is soluble in dilute acid and decomposes in anhydrous alcohol. It can also react with hydrogen to make calcium hydride and calcium amide.
- Molecular Weight: 148.25
- Appearance: Powder
- Melting Point: N/A
- Boiling Point: N/A
- Density: 2.63 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 147.894
Preparation
It can be synthesized when calcium is heated at an elevated temperature in the presence of air, which is represented by the following direct reaction of the elements:
3Ca + N2 → Ca3N2
The calcium nitride formed can be easily identified, as it reacts with moisture or water to produce calcium hydroxide and ammonia:
Ca3N2 + 6H2O → 3Ca(OH)2 + 2NH3
Synthesis and reactions
Calcium nitride is formed along with the oxide, CaO, when calcium burns in the air. It can be produced by direct reaction of the elements:
3 Ca + N2 → Ca3N2
It reacts with water or even the moisture in the air to give ammonia and calcium hydroxide:
Ca3N2 + 6 H2O → 3 Ca(OH)2 + 2 NH3
Like sodium oxide, calcium nitride absorbs hydrogen above 350 °C:
Ca3N2 + 2 H2 → 2 CaNH + CaH2
Recent advances in the use of single crystals for a wide range of applications, particularly in electronic surveillance and detection, have resulted in significant research into the development of improved techniques for growing single-crystalline materials.
Uses
- Ca3N2 can be used for obtaining reactive nitride ions.
- Calcium hydride (a desiccant) can be produced by heating calcium nitride with hydrogen at temperatures above 350°C.
Toxicity
Ca3N2 has been linked to eye damage and skin burns after repeated contact or overexposure. Because of the risk of dangerous reactions, it is a flammable solid that should be kept away from sparks, heat, open flames, and water.