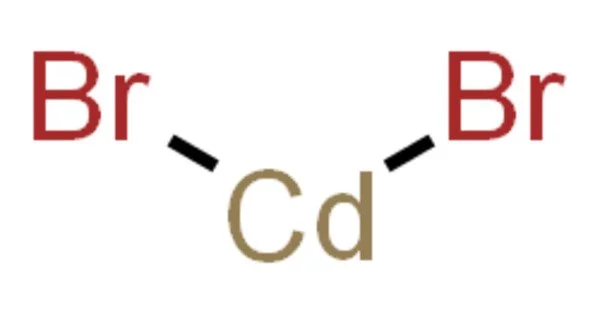
Cadmium bromide is a white to yellowish, odorless, crystalline solid that, when exposed to dry air, transforms into a powder. CdBr2 is the formula for an inorganic compound. It is a hygroscopic white solid. It is also available as a mono- and tetrahydrate. It has only a few applications.
Cadmium bromide is made by heating cadmium in the presence of bromine vapor. The compound can also be synthesized by treating dry cadmium acetate with glacial acetic acid and acetyl bromide. It can also be obtained by dissolving cadmium or cadmium oxide in hydrobromic acid and evaporating the solution to dryness in an inert atmosphere under helium.
Properties
Cadmium bromide is a white to yellowish crystalline powder. It is white to yellowish powder or flakes; hexagonal crystal system; hygroscopic; density 5.192g/cm3; melts at 568°C; vaporizes at 844°C; soluble in water, alcohol, ether, acetone, and liquid ammonia.
- Chemical formula: CdBr2
- Molar mass: 272.22 g/mol
- Appearance: white solid
- Density: 5.192 g/cm3, solid
- Melting point: 568 °C (1,054 °F; 841 K)
- Boiling point: 844 °C (1,551 °F; 1,117 K)
- Solubility in water: 56.3 g/100 mL (0 °C); 160 g/100 mL (100 °C)
- Solubility: soluble in alcohol, ether, acetone and liquid ammonia.
- Crystal structure: Rhombohedral, hr9

Preparation
Cadmium bromide can be made by heating cadmium with bromine vapor or by treating dry cadmium acetate with glacial acetic acid and acetyl bromide. It’s used in photography, engraving, printing, and lithography, among other things.
Uses
This substance is employed in the fields of photography, engraving, and lithography. The other halogen elements combine with cadmium in an ionic reaction similar to bromine. The yellowish crystalline powder is water and alcohol soluble, and slightly soluble in ether. The crystals are delicate and must be kept in a tightly sealed bottle. Cadmium bromide, like its iodide counterpart, was used in collodion in conjunction with an ammonium or potassium iodide.