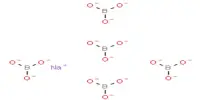
Cadmium azide is an inorganic chemical compound with the formula Cd(N3)2. It is composed of the cadmium cation (Cd2+) and the azide anions (N−3). It is typically used in the production of explosives or pyrotechnics because the azide group is highly reactive, making the compound sensitive to heat, shock, or friction. It is a relatively rare chemical that, like other azides, is considered to be highly explosive under certain conditions. As such, it should be handled with care due to its potential hazards, especially in large quantities.
Properties
Cadmium azide is colorless and crystalline powder. It is highly sensitive to pressure, and is explosive akin to most other azides. It has a high temperature resistance, and also possesses good detonation ability. Because of this, cadmium azide is expected to be applicable within microinitiating systems.
Properties
- Chemical formula: Cd(N3)2
- Molar mass: 196.46 g/mol
- Appearance: colorless
- Solubility: It is moderately soluble in water, but its solubility can depend on factors like temperature and the presence of other ions in the solution.
- Stability: As an azide compound, cadmium azide is sensitive to shock, heat, and friction, which can cause it to detonate. Like other azides, it can decompose explosively when disturbed.
- Chemical Reactivity: It can react with acids to release nitrogen gas and form cadmium salts.
Occurrence
- Natural Occurrence: Cadmium azide is not typically found in nature. It is primarily synthesized in laboratories for research purposes, often in the context of studying explosives or the properties of azide compounds.
- Laboratory Synthesis: Cadmium azide can be synthesized by reacting cadmium salts, such as cadmium chloride, with sodium azide (NaN₃) in a suitable solvent like water or alcohol.
Hazards
Due to its explosive nature, cadmium azide is considered a dangerous material. It’s toxic if ingested, inhaled, or absorbed through the skin, and it can pose serious health risks, particularly because of the cadmium content, which is toxic in its own right.