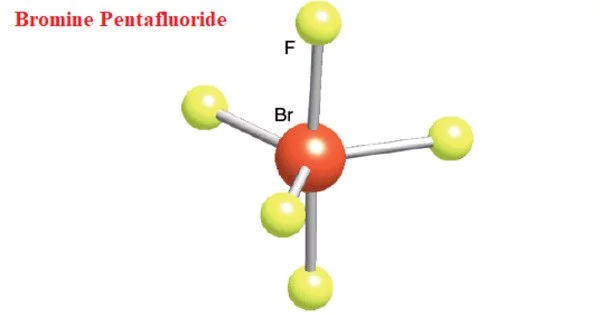
Bromine pentafluoride, (BrF5), is a bromine fluoride and an interhalogen compound. It has a high fluorinating power. It is used in the production of other chemicals as well as rockets. It is extremely toxic when inhaled. It corrodes metals and tissue. It will hasten the combustion of combustible material. If the containers catch fire, they may explode violently and rocket.
BrF5 is used in the analysis of oxygen isotopes. In the presence of bromine pentafluoride, laser ablation of solid silicates releases O2 for subsequent analysis. It has also been used as an oxidizer in liquid rocket propellants and as a fluorinating agent in uranium processing.
Properties
Bromine pentafluoride appears as a colorless, fuming liquid with a pungent odor. At temperatures above boiling point this chemical is a colorless gas.
- Molar mass: 174.894 g.mol−1
- Appearance: Pale yellow liquid
- Density: 2.466 g/cm3
- Melting point: −61.30 °C (−78.34 °F; 211.85 K)
- Boiling point: 40.25 °C (104.45 °F; 313.40 K)
- Solubility in water: Reacts with water
Preparation
Bromine pentafluoride is made by fluorinating bromine at 200 degrees Celsius. The reaction takes place in an iron or copper vessel. Nitrogen is used to dilute the halogens. It was created in 1931 through the direct reaction of bromine and fluorine. This reaction is suitable for large-scale production and is carried out at temperatures above 150 °C (302 °F) with an excess of fluorine:
Br2 + 5 F2 → 2 BrF5
For the preparation of smaller amounts, potassium bromide is used:
KBr + 3 F2 → KF + BrF5
This route yields bromine pentafluoride almost completely free of trifluorides and other impurities.
Reactions
Bromine pentafluoride reacts with water to form bromic acid and hydrofluoric acid (especially when moderated by dilution with acetonitrile):
BrF5 + 3 H2O → HBrO3 + 5 HF
It is an extremely effective fluorinating agent, being able to convert most metals to their highest fluorides even at room temperature. With uranium and uranium compounds, it can be used to produce uranium hexafluoride:
5 U + 6 BrF5 → 5 UF6 + 3 Br2
Hazards
Bromine pentafluoride has a strong corrosive and toxic reaction with water. Its vapors can also be extremely irritating to the eyes, skin, and mucous membranes. When exposed to moist air, it will emit “smoke” containing acidic vapors, similar to chlorine trifluoride, chlorine pentafluoride, and bromine trifluoride, as a result of its reaction with the water in the air. Furthermore, most experimental animals die after being exposed to 100 ppm or higher for more than one minute. Chronic exposure can lead to kidney and liver damage.
When it comes into contact with organic materials or metal dust, it may spontaneously ignite or explode.