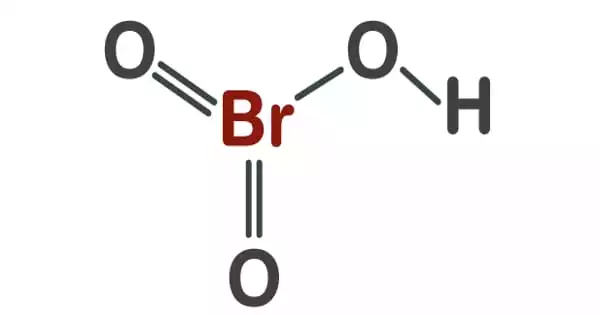
The oxoacid hydrogen bromate has the chemical formula HBrO3. Its salts, the bromates, are strong oxidizing agents in solid form, similar to chlorates, and are utilized in specialized pyrotechnical mixes. It can only be found in aqueous solutions. It is a colorless solution that decomposes to bromine at room temperature and turns yellow. It is a bromate conjugate acid.
Hydrogen Bromate and bromates are strong oxidizing agents that are frequently used in Belousov–Zhabotinsky reactions. Non-equilibrium thermodynamics is exemplified by the Belousov-Zhabotinsky reactions. The color of the solution changes to yellow after its breakdown to bromine at room temperature.
Properties
It is an oxoacid and it exists only in the aqueous solution. Although, it is a solution that has no color, during its decomposition to bromine at room temperature, the color of the solution changes to yellow.
- Molecular weight: 128.91 g/mol
- Conjugate base: Bromate
- pKa: −2
- Complexity: 46.2
Dissociation
Hydrogen bromate is a strong oxidizing agent that is commonly used in the Belousov-Zhabotinsky reaction. Low amounts entirely dissociate to hydronium and bromate, whereas high concentrations breakdown to create bromine. It is produced by the reaction of barium bromate [Ba(BrO3)2] with sulfuric acid (H2SO4). The barium sulfate obtained is in the form of a precipitate.
The extreme instability of hydrogen bromate can be explained by the fact that the positively charged hypervalent bromine is coupled to the electronegative OH group. As a result, it is possible to decant it by removing the precipitated barium sulfate.
It has a number of isomers. It is an oxoacid that only exists as an aqueous solution. It is a colorless solution that turns yellow when exposed to light and decomposes into bromine.
Synthesis
Hydrogen bromate is the product of a reaction of barium bromate and sulfuric acid.
Ba(BrO3)2 + H2SO4 → 2 HBrO3 + BaSO4
Barium sulfate is insoluble in water and forms a precipitate. The aqueous bromic acid can be decanted removing the barium sulfate.
Usages
Hydrogen bromate is useful in the synthesis of bromates. Hydrobromic acid is primarily used in the synthesis of inorganic bromides. Zinc, calcium, and sodium bromides, in particular. It can also be used as a reagent in the synthesis of organobromine compounds. It is also used to catalyze alkylation processes and the extraction of specific ores.
Bromine is a potent oxidizing agent that can liberate oxygen-free radicals from water in mucous membranes. Bromide ions are thought to substitute for chloride ions in the actions of neurotransmitters and transport systems. As a result, it has an impact on a variety of synaptic functions.