Biography of Robert Burns Woodward
Robert Burns Woodward – American organic chemist.
Name: Robert Burns Woodward
Date of Birth: April 10, 1917
Place of Birth: Boston, Massachusetts, United States
Date of Death: July 8, 1979 (aged 62)
Place of Death: Cambridge, Massachusetts, United States
Occupation: Chemist
Father: Arthur Chester Woodward
Mother: Margaret
Spouse/Ex: Irja Pullman (M. 1938), Eudoxia Muller (1946-1972)
Children: Crystal Elisabeth, Eric Richard Arthur, Jean Kirsten, Siiri Anna
Early Life

An American chemist best known for his syntheses of complex organic substances, including cholesterol and cortisone (1951), strychnine (1954), and vitamin B12 (1971), Robert Burns Woodward was born on April 10, 1917, in Boston, Massachusetts, to Margaret (née Burns, an immigrant from Scotland, and a descendant of Robert Burns, the poet) and Arthur Chester Woodward, son of Roxbury, Massachusetts apothecary, Harlow Elliot Woodward. He is considered by many to be the preeminent organic chemist of the twentieth century, having made many key contributions to the subject, especially in the synthesis of complex natural products and the determination of their molecular structure. He also worked closely with Roald Hoffmann on theoretical studies of chemical reactions. He was awarded the Nobel Prize for Chemistry in 1965, “for his outstanding achievements in the art of organic chemistry.”
Brought up singlehandedly by his widowed mother, Woodward studied extensively at home right from his childhood. His habit of extracurricular study almost made him lose his berth at the Massachusetts Institute of Technology. Ultimately, he earned both his bachelor and Ph.D. degree from MIT in just four years. His first work, synthesis of quinotoxine, was completed when he was just twenty-seven years old. Later, he worked mostly on the determination of structure and synthesizing of different natural products. Until his death, he had around two hundred publications to his name, which encompassed the structural determination of complex natural products, syntheses of medicinal compounds, and his theories that linked quantum mechanics and organic chemistry. One unique point of his strategy was that he maintained a close relationship with industry, who often financed his projects. His achievements in the academic field were no less outstanding. He received 26 awards and 45 honorary degrees from established institutions. He also trained around 200 doctoral and postdoctoral students.
Childhood, Family and Educational Life

Robert Burns Woodward was born on April 10, 1917, in Boston, Massachusetts. His father, Arthur Chester Woodward, died of influenza pandemic one year after his birth. Although his mother, Margaret (née Burns), remarried she was soon abandoned by her second husband. Thus, Robert was brought up singlehandedly by his mother.
Woodward’s early years are often told as the story of a boy-genius. He was an autodidact who, even as a child, had a passion for chemistry. At age 14, Woodward bought a copy of Ludwig Gattermann’s Practical Methods of Organic Chemistry and requested issues of chemistry journals from Verlag Chemie of Berlin. Later in life, he did nothing to discourage a persistent legend that he had performed all the experiments in Gattermann’s book.
Woodward began his education at a public primary school. Later, he was admitted to Quincy High School, a public secondary school located in the suburbs of Boston. However, he was mostly an autodidact and read widely at home. His thrust for knowledge was such that, in 1928, he procured chemistry journals from Verlag Chemie through the German Consul-General in Boston. Then by the age of fourteen, he bought Ludwig Gattermann’s Practical Methods of Organic Chemistry and performed all the experiments mentioned in the book on his own.
In 1933, Woodward entered the Massachusetts Institute of Technology (MIT) but neglected his formal studies badly enough to be excluded at the end of the 1934 fall term. MIT readmitted him in the 1935 fall term, and by 1936 he had received the Bachelor of Science degree. Only one year later, MIT awarded him the doctorate, when his classmates were still graduating with their bachelor’s degrees. His doctoral thesis dealt with estrone, which is a female steroid hormone. The research resulted in the publication of several papers in the Journal of the American Chemical Society in 1940.
Personal Life
In 1938, Robert Woodward married Irja Pullman. The couple had two daughters: Siiri Anna (b. 1939) and Jean Kirsten (b. 1944).
In 1946, Woodward married Eudoxia Muller, an artist, and technician whom he met at the Polaroid Corp. This marriage, which lasted until 1972, produced a daughter, and a son: Crystal Elisabeth (b. 1947), and Eric Richard Arthur (b. 1953).
Woodward was a heavy smoker and often lighted his second cigarette from the first. He slept very little and worked from noon till 3 AM in the morning. His rules, which bear his legacy, are sets of empirically derived rules which try to predict the wavelength of the absorption maximum in an ultraviolet-visible spectrum of a specific compound. The Woodward-Hoffmann rules, also named after Robert Burns Woodward and his collaborator Roald Hoffmann, predict the barrier heights of pericyclic reactions based upon conservation of orbital symmetry.
Career and Works
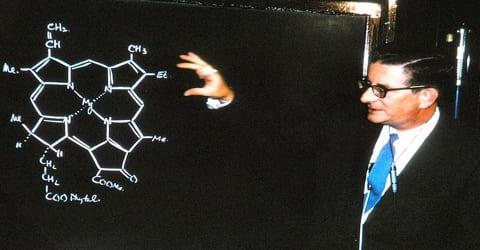
In summer of 1937, Robert Burns Woodward began his career as a postdoctoral fellow at the University of Illinois; but within six months, he shifted to the Harvard University as Junior Fellow. He remained with the Harvard University till his death in 1979. His Fellowship ended in 1938. In the same year, he was accepted as a Member of the Society of Fellow. The position offered him the freedom to pursue his research work independently. On the flip side, he needed collaborators to carry on his experiments, which the position did not allow.
The first major contribution of Woodward’s career in the early 1940s was a series of papers describing the application of ultraviolet spectroscopy in the elucidation of the structure of natural products. Woodward collected together a large amount of empirical data and then devised a series of rules later called the Woodward’s rules, which could be applied to finding out the structures of new natural substances, as well as non-natural synthesized molecules. The expedient use of newly developed instrumental techniques was a characteristic Woodward exemplified throughout his career, and it marked a radical change from the extremely tedious and long chemical methods of structural elucidation that had been used until then.
Therefore in 1941, Woodward accepted the position of the Instructor of Chemistry. Around this time, Woodworth published a few important papers on the correlation between ultraviolet spectra and structure. It later led to the formation of ‘Woodward’s Rules’. However, it was not yet sure if he would have any long term engagement at Harvard and so he considered shifting to California Institute of Technology, Pasadena or the University of California, Barkley. But, he did not have to make any such move; opportunity came from an unusual source.
Woodward was known among his colleagues for his aggressive use of the latest analytic tools. He strongly believed in the utility of instruments such as spectrophotometers in organic synthesis. Such instruments could routinely assist the chemist in the characterization of compounds, and they suggested new generalizations about the relationship of structure to physical properties. Indeed, Woodward’s early theoretical pursuits centered on the use of two types of physical data ultraviolet absorption (1941-42) and optical rotatory dispersion (1961). Both of these generalizations about spectra and structure created new utility for routine spectroscopic measurements. These instrumental techniques altered the traditional, complementary relationship between synthesis and structural determination and reduced the latter to a relatively commonplace procedure.
In 1942, Edwin Land, the founder and head of the Polaroid Corporation, offered him the opportunity to work on quinine. It was a key ingredient for the production of their light polarizing sheets and films, but its supply was affected by the ongoing Second World War. In the same year, Woodworth created a chemically simple, light-polarizing replacement for quinine. Afterward, he asked Land to support him for synthesizing quinine. The work was started in February 1943. Building on Paul Rabe’s 1908 work, Woodworth and his collaborators completed the synthesis of their key intermediate, quinotoxine, on 10 April 1944. It made him internationally famous and acted as a catalyst in his career.
In 1944, with his postdoctoral researcher, William von Eggers Doering, Woodward reported the synthesis of the alkaloid quinine, used to treat malaria. Although the synthesis was publicized as a breakthrough in procuring the hard to get medicinal compound from Japanese occupied southeast Asia, in reality, it was too long and tedious to adopt on a practical scale. Nevertheless, it was a landmark for chemical synthesis. Woodward’s particular insight in this synthesis was to realize that the German chemist Paul Rabe had converted a precursor of quinine called quinotoxine to quinine in 1905.
During World War II, Woodward worked on the structural elucidation of penicillin, and he and William Doering sought synthetic routes to quinine. In 1948 Woodward published the structure of strychnine, beating English chemist Robert Robinson in the competition to solve this difficult chemical puzzle. During the 1950s, Woodward collaborated with the pharmaceutical company Pfizer, Inc., on the structural analysis of a new series of antibiotics: terramycin, aureomycin, and magnamycin.
During the late 1940s, Woodward synthesized many complex natural products including quinine, cholesterol, cortisone, strychnine, lysergic acid, reserpine, chlorophyll, cephalosporin, and colchicine. With these, Woodward opened up a new era of synthesis, sometimes called the ‘Woodwardian era’ in which he showed that natural products could be synthesized by careful applications of the principles of physical organic chemistry, and by meticulous planning.
In 1950, Woodward became a full professor and in 1951, he was able to synthesize cortisone and cholesterol. At that time, many other scientists were working on the cortisone, vying with each other to be the first to synthesize this ‘miracle drug’; ultimately it was Woodworth, who won the race.
Nevertheless, Woodward’s achievements in the field of structure determination remain milestones in organic chemistry: penicillin (1945), patulin (1948), strychnine (1947), ferrocene (1952), cevine (1954), gliotoxin (1958), ellipticine (1959), calycanthine (1960), oleandomycin (1960), streptonigrin (1963), and tetrodotoxin (1964). With the American biochemist Konrad Bloch, he also first proposed the correct biosynthetic pathway to the steroid hormones in living organisms. Woodward undertook and completed one of the first total syntheses of the steroids cholesterol and cortisone (1951) and then the related terpene lanosterol (1954).
In 1953, Woodward was elected as the Morris Loeb Professor of Chemistry at Harvard University, a position he held till 1960. Also, in 1953, he determined the structure of terramycin. Next, in 1954, he determined the structure of strychnine and lanosterol and also synthesized these two products. His work on strychnine was also done under immense international competition. Subsequently, in 1956, he determined the structure of reserpine and also synthesized the product. It is said to be his first major work, which not only solved the problem of the shortage of raw material but also led to its industrial production. From 1958 to 1964, Woodward worked on gliotoxin, ellipticine, calycanthine, oleandomycin, streptonigri, and tetrodotoxin, successfully determining their structure. These works remain a milestone in the field of organic chemistry.
In 1954 syntheses of strychnine and lysergic acid were announced, followed in 1956 by a synthesis of reserpine that has become a model of elegant technique and has been used for the commercial production of this tranquilizer. Subsequent achievements included the synthesis of chlorophyll (1960), tetracycline (1962), colchicine (1963), and cephalosporin C (1965). In a large-scale collaboration with Albert Eschenmoser of the Federal Institute of Technology in Zürich, Woodward completed in 1971 the synthesis of the complicated coenzyme vitamin B12 (cyanocobalamin) by a sequence of more than 100 reactions. The work on vitamin B12 led to the recognition and formulation, with the American chemist Roald Hoffmann, of the concept of conservation of orbital symmetry, explicating a broad group of fundamental reactions.
In the early 1950s, Woodward, along with the British chemist Geoffrey Wilkinson, then at Harvard, postulated a novel structure for ferrocene, a compound consisting of a combination of an organic molecule with iron. This marked the beginning of the field of transition metal organometallic chemistry which grew into an industrially very significant field. Wilkinson won the Nobel Prize for this work in 1973, along with Ernst Otto Fischer. Some historians think that Woodward should have shared this prize along with Wilkinson. Remarkably, Woodward himself thought so and voiced his thoughts in a letter sent to the Nobel Committee. Woodward won the Nobel Prize in 1965 for his synthesis of complex organic molecules. He had been nominated a total of 111 times from 1946 to 1965. In his Nobel lecture, he described the total synthesis of the antibiotic cephalosporin and claimed that he had pushed the synthesis schedule so that it would be completed around the time of the Nobel ceremony.
In 1963, Woodward became the Donner Professor of Science and at the same time, he assumed the dual responsibility of directing the Woodward Research Institute at Basel. Also in the 1960s, he worked as Consultant for Polaroid’s development of color photographic processes. His second major work, which concerned the synthesis of Vitamin B-12, also began in the early 1960s. In this work, he collaborated with Albert Eschenmoser of Zurich. A team of almost 100 students and postdoctoral fellows worked for years on this project before it was synthesized in 1973.
Woodward lived between the worlds of academe and industry. During his career, he held consultancies with Eli Lilly and Company, Merck & Co., Inc., Mallinckrodt Pharmaceuticals, Monsanto Company, Polaroid Corporation, and Pfizer. In 1963 Ciba (later Ciba-Geigy Ltd., now Novartis International AG), a Swiss pharmaceutical firm, set up the Woodward Research Institute in Basel. He then held dual appointments as director of the institute and as Donner Professor of Science at Harvard. Between Basel and Cambridge, more than 400 graduate and postdoctoral students trained in Woodward’s laboratories.
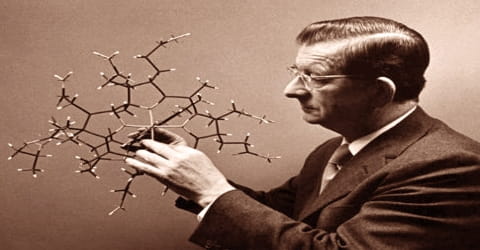
Also in 1973, based on observations made during the B12 synthesis, he and Roald Hoffmann devised rules for elucidating the stereochemistry of the products of organic reactions. It is now known as Woodward-Hoffmann rules. However, he did not stop there but continued to work until his end. The synthesis included almost a hundred steps and involved the characteristic rigorous planning and analyses that had always characterized Woodward’s work. This work, more than any other, convinced organic chemists that the synthesis of any complex substance was possible, given enough time and planning (see also palytoxin, synthesized by the research group of Yoshito Kishi, one of Woodward’s postdoctoral students). As of 2016, no other total synthesis of Vitamin B12 has been published.
At the time of his death in 1979, Woodward was working on the synthesis of erythromycin. Apart from his research work, Woodward authored/co-authored more than 200 publications and trained more or less the same number of doctoral or post-doctoral students, many of whom went on to become distinguished academics later.
Awards and Honor
For his work, Robert Burns Woodward received many awards, honors and honorary doctorates, including election to the National Academy of Sciences in 1953, and membership in academies around the world.
In 1958, Woodward was elected as a Foreign Member of the Royal Society, London.
In 1965, Robert Burns Woodward was awarded the Nobel Prize in Chemistry “for his outstanding achievements in the art of organic synthesis”. From 1966 to 1971, he was a member of the Corporation of the Massachusetts Institute of Technology.
Death and Legacy
Robert Burns Woodward died on July 8, 1979, from a heart attack at Cambridge, Massachusetts.
Woodward had an encyclopedic knowledge of chemistry and an extraordinary memory for detail. Probably the quality that most set him apart from his peers was his remarkable ability to tie together disparate threads of knowledge from the chemical literature and bring them to bear on a chemical problem.
Synthesis of reserpine is taken as Woodward’s first major work. Earlier, the natural product was imported from India to be used as a sedative. Synthesizing the product has not only made it more readily available but has also brought in radical changes in the treatment of mental illness. Synthesizing the complicated coenzyme Vitamin B12 (cyanocobalamin) is another of his major work. Done in collaboration with Albert Eschenmoser of the Federal Institute of Technology in Zürich, the work is taken as a landmark in the history of organic chemistry.
Woodward had an enormous capacity for information and superb mental organization. Given the set of data on a structure or the planning of a synthesis, Woodward brought to bear a most remarkable ability to see the entire problem at once and to solve it systematically. His brilliance lay in the quality and depth of his thought, his painstaking preparations, and his chemical intuition. Woodward’s work was central to the chemical thought of the times, and his influence on other organic chemists was arguably greater than that of any other in his era.
For his contribution to Woodward-Hoffmann rules, Hoffmann received the 1981 Nobel Prize in Chemistry (shared with Kenichi Fukui). Had Woodward been alive till then, there is no doubt that he would have received the Nobel Prize for the second time.
Information Source: