Barium titanate (BTO) is a chemical substance having the formula BaTiO3. When formed as big crystals, barium titanate appears white as a powder and is transparent. It’s a ferroelectric ceramic material with photorefractive and piezoelectric characteristics. It dissolves in a wide range of acids, including sulfuric, hydrochloric, and hydrofluoric acids. It is insoluble in acids and bases, as well as in water. Capacitors, electromechanical transducers, and nonlinear optics all make use of it.
Properties
The comparatively easy sol–hydrothermal process can be used to produce barium titanate. Heating barium carbonate and titanium dioxide can also produce barium titanate. The reaction is carried out by liquid phase sintering. Single crystals of molten potassium fluoride can be formed at roughly 1100 °C.
- Appearance: transparent large crystals and as a white powder
- Molar Mass: 233.192 g/mol.
- Density: 6.02 g/cm3 in solid state and 6.08 g/mL in liquid state at 25 °C.
- Melting Point: 1625 °C.
- Hardness: 5 in Mohs scale.
Production
Titanium dioxide and barium carbonate are heated to produce barium titanate. This reaction occurs via liquid phase sintering. Single crystals of Barium titanate can be formed from molten potassium fluoride at temperatures of 1100 °C.

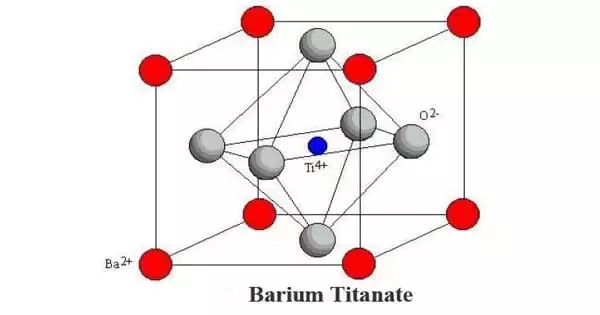
Structure
Depending on the temperature, the solid occurs in one of four polymorphs. From high to low temperatures, the four polymorphs’ crystal symmetries are cubic, tetragonal, orthorhombic, and rhombohedral. Except for the cubic phase, all of these phases exhibit the ferroelectric effect. The high-temperature cubic phase is the simplest to describe since it is made up of regular corner-sharing octahedral TiO6 units that construct an O-verticed cube with Ti-O-Ti edges.
With a notional coordination number of 12, Ba2+ is positioned at the center of the cube in the cubic phase. Lower symmetry phases, which include Ti4+ migration to off-center locations, are stable at lower temperatures. The amazing features of this material result from the Ti4+ distortions’ cooperative activity.
Solubility
In water and alkalis, barium titanate is insoluble. However, it is soluble in a number of acids, including hydrochloric acid, hydrofluoric acid, and sulfuric acid. In concentrated hydrofluoric acid and sulfuric acid, the chemical dissolves entirely.
Uses
With dielectric constant values as high as 7,000, barium titanate is a dielectric ceramic used in capacitors. Values as high as 15,000 are achievable over a restricted temperature range; most popular ceramic and polymer materials have values less than 10, while others, such as titanium dioxide (TiO2), have values between 20 and 70.
In its purest form, barium titanate can be utilized as an electrical insulator. As a dielectric ceramic material, the compound is employed in capacitors. It is also utilized in microphones and other transducers as a piezoelectric material.