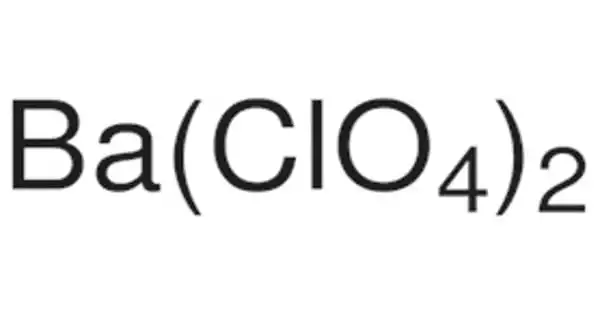With the formula Ba(ClO4)2, barium perchlorate is a potent oxidizer. It appears as a crystalline white solid. Noncombustible, but it hastens the combustion of combustible materials. It is commonly used in the pyrotechnic industry. If there are large quantities involved in a fire or the combustible material is finely divided, it may explode.
At 505 °C, barium perchlorate degrades.
Properties
- Molecular Weight: 336.24
- Appearance: White powder, granules, or chunks
- Melting Point: 505 °C
- Boiling Point: N/A
- Density: 3.20 g/cm3
- Solubility in H2O: N/A
Structure of barium perchlorate trihydrate
Gallucci and Gerkin (1988) analyzed the structure of the hydrate isomer barium perchlorate trihydrate (Ba(ClO4)2•3H2O) by X-ray crystallography. The barium ions are coordinated by six water oxygen atoms at 2.919Å and six perchlorate oxygens at 3.026Å in a distorted icosahedral arrangement. The perchlorate fails by a narrow margin to have regular tetrahedral geometry and has an average Cl-O bond length of 1.433Å.
The space-group assignment of the structure was resolved, with the centrosymmetric assignment of P63/m confirmed. Each axial perchlorate oxygen is hydrogen-bonded to three water molecules and each trigonal oxygen is hydrogen bonded to two water molecules. This interaction is the reason that the perchlorate fails to be tetrahedral. Gallucci and Gerkin surmised that the water molecule H atoms lie in the plane at z = 1⁄4 and 3⁄4.

Preparation
Many different reagents and procedures can be used to make barium perchlorate. Evaporating a solution containing barium chloride and an excess of perchloric acid is one way. Recrystallizing and drying to a fixed weight yields the dihydrate form. The monohydrate is obtained after further drying over sulfuric acid. The anhydrous form is produced by vacuum heating at 140 °C.
Dehydration of barium perchlorate that does not occur in a vacuum will result in perchlorate hydrolysis. Other reactions that result in barium perchlorate include perchloric acid and barium hydroxide or carbonate; potassium perchlorate and hydrofluosilicic acid followed by barium carbonate; and a boiling solution of potassium chlorate and zinc fluosilicate. Barium perchlorate is produced on a large scale by evaporating a solution of sodium perchlorate and barium chloride. Another way is to digest a saturated solution of ammonium perchlorate with hydrated barium hydroxide in 5-10% excess of the theoretical amount.
Applications
One of the principal applications of barium perchlorate is in the creation and preparation of explosive emulsions and other explosive compounds due to its property as a powerful oxidation agent. The use of an emulsifier simplifies the process of transporting and handling explosive materials while keeping their destructive qualities at the end point of usage. During the 1920s, perchlorate explosives were mostly utilized in industrial applications such as mining.
Barium perchlorate can also form complexes with the quinolone antibiotics ciprofloxacin and norfloxacin. According to FTIR data, CIP and NOR work as bidentate ligands by using the ring carbonyl oxygen and a carboxylic group oxygen. This coordination is important because it boosts the antibiotics’ solubility in water and other polar solvents, hence enhancing their absorption efficiency.