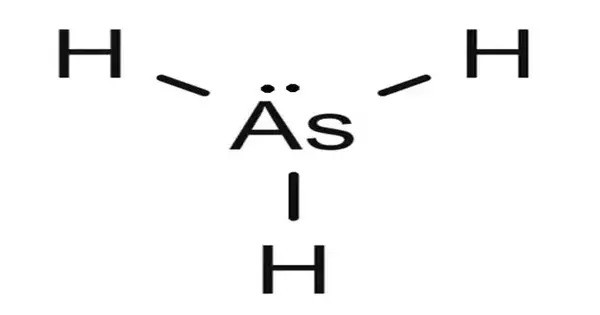
An arsenide hydride or hydride arsenide is a chemical compound containing hydride (H−) and arsenide (As3−) ions in a single phase. They are in the class of mixed anion compounds. It is a colorless, flammable, and highly toxic gas with a faint garlic-like odor. It consists of one arsenic atom bonded to three hydrogen atoms, forming a trigonal pyramidal structure. Arsine is heavier than air and slightly soluble in water, with a boiling point of -62.5°C.
Arsine is primarily used in the semiconductor industry for doping silicon-based materials and in the synthesis of organoarsenic compounds. It is produced industrially by reducing arsenic compounds, such as arsenic trichloride, with hydrogen or metal hydrides, or as a byproduct in certain metal refining processes.
Historically, arsine poisoning was linked to industrial accidents or chemical warfare research. Safe handling requires strict precautions, including ventilation and gas detection systems. While not naturally abundant, arsine’s risks demand careful management in industrial settings.
Preparation
Arsenide hydride compounds may be produced by heating metal hydride and metal arsenide mixtures under pressure.
Exposure to arsine is extremely dangerous, as it can cause severe health effects, including rapid hemolysis (destruction of red blood cells), leading to kidney failure, jaundice, and potentially death at low concentrations (0.5–10 ppm). Symptoms may be delayed, complicating diagnosis. Its toxicity stems from its ability to bind to hemoglobin, disrupting oxygen transport.
Properties
Arsenide hydrides are under investigation as unconventional superconductors. The hydride ions can be replaced by fluoride or oxide ions to yield variations in composition.
- Appearance: Colorless, flammable gas with a faint garlic-like or fishy odor at low concentrations.
- Molecular Weight: 77.95 g/mol.
- Boiling Point: −62.5°C (−80.5°F).
- Melting Point: −116°C (−177°F).
- Density: 3.186 g/L at 0°C and 1 atm (denser than air).
- Solubility: Slightly soluble in water (0.2 g/L at 20°C), more soluble in organic solvents like benzene or chloroform.
Chemical Properties
- Reactivity: Highly reactive, unstable at elevated temperatures, decomposing into arsenic and hydrogen gas. It reacts with oxygen, halogens, and strong oxidants, often leading to toxic arsenic compounds.
- Toxicity: Extremely toxic, even at low concentrations (0.5 ppm can be lethal). It causes severe health effects, including hemolysis (destruction of red blood cells), kidney failure, and death in high exposure.
- Stability: Thermally unstable, decomposes above 230°C. It is sensitive to light and moisture, which can catalyze decomposition.
Natural Occurrence
- Rare in Nature: Arsine is not commonly found in the environment due to its instability. However, it can form in trace amounts through microbial reduction of arsenic-containing compounds in anaerobic environments, such as wetlands or sediments rich in arsenic.
- Geological Context: Associated with arsenic-bearing minerals (e.g., arsenopyrite, realgar) when they react with water or acids under specific conditions.
Safety and Handling
- Hazards: Highly flammable and toxic. Exposure limits are stringent (OSHA PEL: 0.05 ppm). Symptoms of exposure include headache, nausea, abdominal pain, and hemoglobinuria (dark urine).
- Precautions: Use in well-ventilated areas with gas detection systems. Personal protective equipment (PPE) and strict handling protocols are essential in industrial settings.