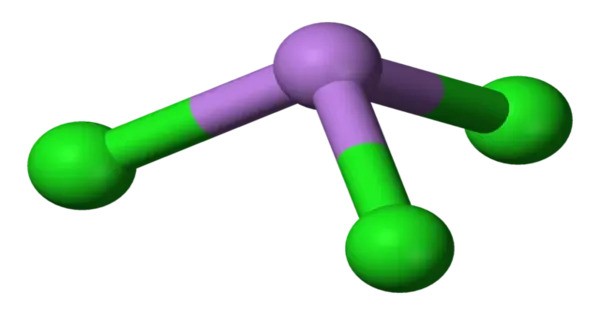
Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. It is a toxic and corrosive substance that is typically a colorless liquid, but it can also appear as a white crystalline solid at lower temperatures. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.
Properties
- Chemical formula: AsCl3
- Molar mass: 181.28 g/mol
- Appearance: colourless oily liquid
- Density: 2.163 g/cm3, liquid
- Melting point: −16.2 °C (2.8 °F; 256.9 K)
- Boiling point: 130.2 °C (266.4 °F; 403.3 K)
- Solubility in water: Hydrolyzes
- Solubility: soluble in alcohol, ether, HCl, HBr, chloroform, CCl4
Synthesis
This colourless liquid is prepared by treatment of arsenic(III) oxide with hydrogen chloride followed by distillation: As2O3 + 6 HCl → 2 AsCl3 + 3 H2O
It can also be prepared by chlorination of arsenic at 80–85 °C, but this method requires elemental arsenic.
2 As + 3 Cl2 → 2 AsCl3
Arsenic trichloride can be prepared by the reaction of arsenic oxide and sulfur monochloride. This method requires simple apparatus and proceeds efficiently:
2 As2O3 + 6 S2Cl2 → 4 AsCl3 + 3 SO2 + 9 S
A convenient laboratory method is refluxing arsenic(III) oxide with thionyl chloride:
2 As2O3 + 3 SOCl2 → 2 AsCl3 + 3 SO2
Arsenic trichloride can also be prepared by the reaction of hydrochloric acid and arsenic(III) sulfide.
As2S3 + 6 HCl → 2 AsCl3 + 3 H2S
Reactions
Hydrolysis gives arsenous acid and hydrochloric acid:
AsCl3 + 3 H2O → As(OH)3 + 3 HCl
Although AsCl3 is less moisture sensitive than PCl3, it still fumes in moist air.
AsCl3 undergoes redistribution upon treatment with As2O3 to give the inorganic polymer AsOCl. With chloride sources, AsCl3 also forms salts containing the anion [AsCl4]−. Reaction with potassium bromide and potassium iodide give arsenic tribromide and arsenic triiodide, respectively.
AsCl3 is useful in organoarsenic chemistry, for example triphenylarsine is derived from AsCl3:
AsCl3 + 6 Na + C6H5Cl → As(C6H5)3 + 6 NaCl
The chemical weapons called Lewisites are prepared by the addition of arsenic trichloride to acetylene:
AsCl3 + C2H2 → ClCH=CHAsCl2
Production
Arsenic trichloride is typically produced by reacting arsenic (in the form of arsenic pentoxide or arsenic metal) with chlorine gas:
As2O3 + 3Cl2 → 2AsCl 3 + 3O 2
It can also be made by reacting arsenic with chlorine gas at elevated temperatures.
Uses
- Synthesis of other compounds: Arsenic trichloride is used as an intermediate in the production of various other arsenic compounds, such as gallium arsenide (GaAs), used in semiconductors.
- Wood preservation: It has been historically used in wood treatment to prevent decay, though its use has decreased due to health and environmental concerns.
- Catalysis: It can act as a catalyst in organic synthesis, particularly in the preparation of other organoarsenic compounds.
Safety and Hazards
- Toxicity: Arsenic trichloride is highly toxic and a known carcinogen. Inhalation or ingestion can lead to severe poisoning. Chronic exposure can cause skin lesions, lung cancer, and other serious health issues.
- Corrosive nature: It is corrosive to skin and eyes, and contact should be avoided.
- Reactivity: It is highly reactive with water, releasing toxic fumes of hydrogen chloride (HCl) and arsenic oxides. It should be handled with care in a controlled environment.