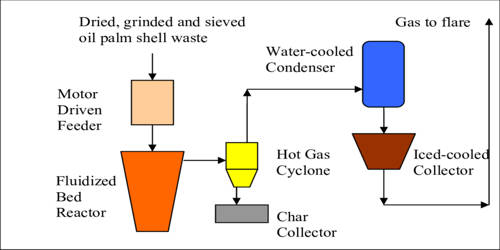
Pyrolysis is the thermal decomposition of materials at elevated temperatures in an inert atmosphere. It is a process of chemically decomposing organic materials at elevated temperatures in the absence of oxygen. It involves a change in chemical composition. The process typically occurs at temperatures above 430 °C (800 °F) and under pressure.
Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood. In general, the pyrolysis of organic substances produces volatile products and leaves a solid residue enriched in carbon, char. It simultaneously involves the change of physical phase and chemical composition and is an irreversible process.
Pyrolysis is commonly used to convert organic materials into a solid residue containing ash and carbon, small quantities of liquid and gases. The process is used heavily in the chemical industry, for example, to produce ethylene, many forms of carbon, and other chemicals from petroleum, coal, and even wood, to produce coke from coal. Aspirational applications of pyrolysis would convert biomass into syngas and biochar, waste plastics back into usable oil, or waste into safely disposable substances.
Terminology
Pyrolysis is one of the various types of chemical degradation processes that occur at higher temperatures (above the boiling point of water or other solvents). It differs from other processes like combustion and hydrolysis in that it usually does not involve the addition of other reagents such as oxygen (O2, in combustion) or water (in hydrolysis).
Types of pyrolysis
Complete pyrolysis of organic matter usually leaves a solid residue that consists mostly of elemental carbon; the process is then called carbonization. More specific cases of pyrolysis include:
- dry distillation, as in the original production of sulfuric acid from sulfates
- destructive distillation, as in the manufacture of charcoal, coke and activated carbon
- caramelization of sugars
- high-temperature cooking processes such as roasting, frying, toasting, and grilling
- charcoal burning, the production of charcoal
- tar production by pyrolysis of wood in tar kilns
- cracking of heavier hydrocarbons into lighter ones, as in oil refining
- hydrous pyrolysis, in the presence of superheated water or steam, also used in oil refining
- catagenesis, the natural conversion of buried organic matter to fossil fuels and
- flash vacuum pyrolysis, used in organic synthesis.
Advantages of Pyrolysis –
- It is a simple, inexpensive technology for processing a wide variety of feedstocks.
- It reduces waste going to landfill and greenhouse gas emissions.
- It reduces the risk of water pollution.
- Waste management with the help of modern pyrolysis technology is inexpensive than disposal to landfills.
- The construction of a pyrolysis power plant is a relatively rapid process.