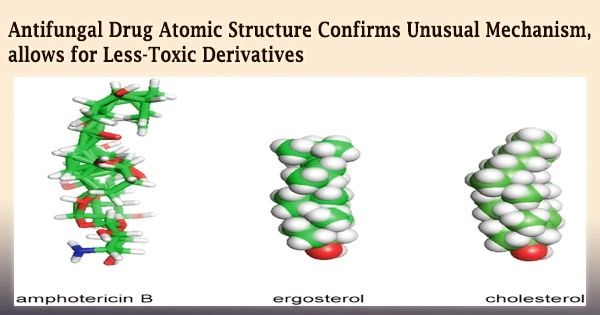
Advanced molecular imaging technology has finally traced the structure of a medicine that has baffled researchers and medics for nearly 70 years.
Researchers from the University of Illinois Urbana-Champaign, the University of Wisconsin, Madison, and the National Institutes of Health have detailed the structure of amphotericin B, a strong but lethal antifungal medication, in atomic detail in a new study.
Dr. Martin D. Burke, a professor of chemistry at Illinois and a member of the Carle Illinois College of Medicine, as well as a medical practitioner, said that seeing the structure helps the researchers develop less harmful AmB derivatives.
Burke collaborated on the study alongside Chad Rienstra, a biochemistry professor from Wisconsin, and Taras Pogorelov, a chemical research professor from Illinois. The findings were published in the journal Nature Structural & Molecular Biology.
“It’s like we were driving in the dark at night, and all of a sudden we were able to put the lights on. With the clarity of this structure, we can see where we need to go to reach our goal of a less-toxic antifungal drug,” Burke said.
Researchers and doctors previously believed that AmB killed fungal cells by generating channels in the cell membrane, the cell’s outer envelope.
However, Burke and Rienstra’s research discovered in 2014, while Rienstra was a professor at Illinois, that amphotericin kills cells primarily by robbing the membrane of sterol components cholesterol in human cells and ergosterol in fungal cells.
Individual amphotericin molecules clumped together to form a bigger structure that, like a sponge, absorbed sterol molecules from cell membranes, killing the cells.
We are already in the process of investigating the structures of the AmB complexes with both cholesterol and ergosterol. It opens the door to finally build or find nontoxic derivatives of this important drug and help a lot of people without the horrible side effects that AmB has right now.
Agnieszka Lewandowska
“The ion channel is a secondary action to the antifungal activity. That let us disconnect the ion channel-forming function from the fungicidal activity of amphotericin,” Burke said. His group has applied the channel-forming abilities of AmB as a “molecular prosthetics” approach to treat cystic fibrosis, yet a greater understanding of the fungicidal sterol sponge remained elusive.
“We had some images but no details,” said Agnieszka Lewandowska, a senior research scientist at Illinois and first author of the new study. “Now we can really see the part of the structure that we think is responsible for interacting with cholesterol, which we don’t want. So then we could modify that and make sure it only interacts with ergosterol, which we do want.”
The conventional molecular imaging techniques, such as nuclear magnetic resonance, are challenging to apply because AmB forms a huge aggregate. The researchers used an advanced molecular imaging technology termed magic-angle spinning solid-state NMR and devised innovative sample preparation protocols in the new study.
They also visualized the structures represented by the NMR data using powerful computational modeling approaches. The result was a representation showing how small AmB molecules fit together in a head-to-tail configuration, staggered into a huge lattice, leaving a void shaped and sized just right for sterol molecules in atomistic detail.
The aggregate also had some flexibility, maybe allowing it to stretch a little to accommodate cholesterol, which is slightly larger than ergosterol.
“We wanted to know how the AmB sponge fits together to accommodate ergosterol,” Rienstra said. “Just like sponges that absorb water, if it’s dried out and crusty, it doesn’t move well and won’t do a very good job of absorbing sterols. Once it’s a little soft, it does a better job of absorbing because then it’s flexible.”
According to the experts, the precise structure verifies previous work and also gives a roadmap for building variants.
“We are already in the process of investigating the structures of the AmB complexes with both cholesterol and ergosterol. It opens the door to finally build or find nontoxic derivatives of this important drug and help a lot of people without the horrible side effects that AmB has right now,” Lewandowska said.
Following that, the researchers intend to work together to synthesize derivatives and then study their atomistic structures to see how they aggregate and interact with cholesterol and ergosterol, as well as to investigate the possibilities of additional small molecules.
“Amphotericin works differently than any other drug we know about. It doesn’t bind to a protein; it self-assembles into this interesting aggregate,” Burke said.
“We saw this whole new area of small molecule interactions. This imaging technique is giving us new tools to understand small molecule interactions and how they can perform higher order, proteinlike functions. We’re finally in a position where we can rationally tap into AmB’s huge functional potential, both for antifungal treatment and for molecular prosthetics.”
The National Institutes of Health supported this work.