Organic Compound
Definition
Organic Compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. The few carbon-containing compounds not classified as organic include carbides, carbonates, and cyanides. This is the theory that certain compounds could be synthesized only from their classical elements—earth, water, air, and fire—by the action of a “life-force” (vis vitalis) that only organisms possessed. Vitalism taught that these “organic” compounds were fundamentally different from the “inorganic” compounds that could be obtained from the elements by chemical manipulation.
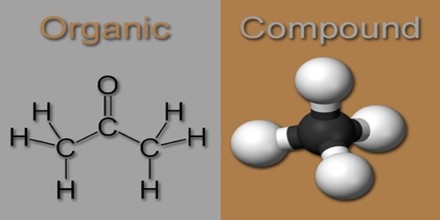
Organic compounds can be found in nature or they can be synthesized in the laboratory. An organic substance is not the same as a “natural” substance. A natural material means that it is essentially the same as it was found in nature, but “organic” means that it is carbon based. The organic compound L-isoleucine molecule presents some features typical of organic compounds: carbon–carbon bonds, carbon–hydrogen bonds, as well as covalent bonds between carbon to oxygen and to nitrogen.
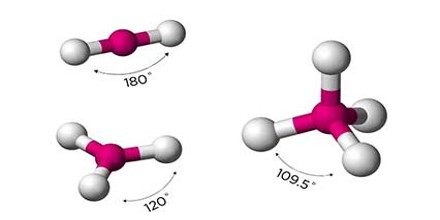
Types of Organic Compound
Organic Compounds, which are the compounds associated with life processes, are the subject matter of organic chemistry. Among the numerous types of organic compounds, four major categories are found in all living things: carbohydrates, lipids, proteins, and nucleic acids.
Carbohydrates –
Almost all organisms use carbohydrates as sources of energy. In addition, some carbohydrates serve as structural materials. Carbohydrates are molecules composed of carbon, hydrogen, and oxygen; the ratio of hydrogen atoms to oxygen and carbon atoms is 2:1.
Simple carbohydrates commonly referred to as sugars, can be monosaccharides if they are composed of single molecules, or disaccharides if they are composed of two molecules. The most important monosaccharide is glucose, a carbohydrate with the molecular formula C6H12O6. Glucose is the basic form of fuel in living things.
Lipids –
Lipids are organic molecules composed of carbon, hydrogen, and oxygen atoms. The ratio of hydrogen atoms to oxygen atoms is much higher in lipids than in carbohydrates. Lipids include steroids, the material of which many hormones are composed, waxes, and fats. The fats in adipose tissue contain much concentrated energy. Hence, they serve as a reserve energy supply to the organism. The enzyme lipase breaks down fats into fatty acids and glycerol in the human digestive system.
Proteins –
Proteins, among the most complex of all organic compounds, are composed of amino acids, which contain carbon, hydrogen, oxygen, and nitrogen atoms. Certain amino acids also have sulfur atoms, phosphorus, or other trace elements such as iron or copper.
Proteins are the major molecules from which living things are constructed. Certain proteins are dissolved or suspended in the watery substance of the cells, while others are incorporated into various structures of the cells. Proteins are also found as supporting and strengthening materials in tissues outside of cells. Bone, cartilage, tendons, and ligaments are all composed of proteins.
Nucleic Acids –
The nucleic acids are composed of smaller units called nucleotides. Each nucleotide contains a carbohydrate molecule (sugar), a phosphate group, and a nitrogen-containing molecule that, because of its properties, is a nitrogenous base.
Living organisms have two important nucleic acids. One type is deoxyribonucleic acid, or DNA. The other is ribonucleic acid, or RNA. DNA is found primarily in the nucleus of the cell, while RNA is found in both the nucleus and the cytoplasm, a semiliquid substance that composes the volume of the cell.
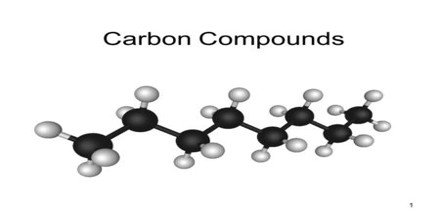
Classification of Organic Compounds
Organic Compounds classified in a variety of ways. One major distinction is between natural and synthetic compounds. It can also be classified or subdivided by the presence of heteroatoms, e.g., organometallic compounds, which feature bonds between carbon and a metal, and organophosphorus compounds, which feature bonds between carbon and a phosphorus.
- Acyclic or Open Chain Compounds: These compounds are also known as aliphatic compounds, they have branched or straight chains.
- Alicyclic or Closed Chain or Ring Compounds: These are cyclic compounds which contain carbon atoms connected to each other in a ring (homocyclic). When atoms other than carbon are also present then it is called as heterocyclic.
- Aromatic Compounds: They are a special type of compounds which contain benzene and other ring related compounds. Similar to alicyclic, they can also have hetero atoms in the ring. Such compounds are called as heterocyclic aromatic compounds.
- Heterocyclic Compounds: In these compounds generally one or more atoms of elements such as nitrogen ‘N’, oxygen ‘O’, or sulphur ‘S’ are present. The atom other than that of carbon viz., N, O or S, present in the ring is called hetero atom. Heterocyclic compounds with five and six atoms in the ring are termed as five-membered and six-membered heterocycles respectively.