According to a new study by Tokyo Tech scientists, the biofilm-forming bacteria E. coli clings strongly to self-assembling monolayers (SAMs) that are hydrophobic and hydrophilic protein-adsorbing and weakly to those that are hydrophilic protein-resisting.
The creation of bacteria-resistant surfaces and antibiofouling coatings for biomedical and industrial equipment may be influenced by the discoveries made regarding how surface chemistry might affect bacterial cell adherence, which in turn affects the formation of biofilms.
Bacterial biofilms are like a double-edged sword. On the one hand, they have proven useful for producing energy and degrading hazardous substances in soil and water, but on the other hand, they are an annoyance when they build up within drinking water pipes, on contact lenses, and on biomedical devices.
Bacterial cells first connect to materials’ surfaces via weak, reversible bonds before becoming biofilms. When these linkages reach maturity, the cells release polymeric materials that produce stable, permanent biofilms.
The key to unlocking biofilm prevention technology is understanding the process underlying irreversible biofilm development and its relationship to surface chemistry. The sorts of surfaces that bacterial cells are most likely to attach to have been narrowed down through numerous methods suggested by scientists over time. Unfortunately, the influence of surface chemistry and surface topography on biofilm formation has remained ambiguous.
Against this backdrop, a team of researchers led by Associate Professor Tomohiro Hayashi from Tokyo Institute of Technology (Tokyo Tech), Japan, set out to address this knowledge gap.
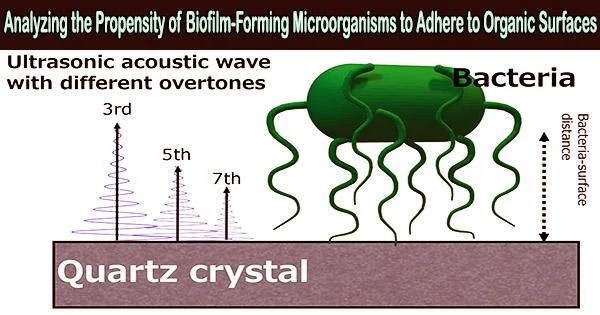
In a recent breakthrough published in ACS Applied Bio Materials, the team investigated the adhesion mechanism of the E. coli bacteria on self-assembling monolayers (SAM) comprising different terminal groups, i.e., different surface chemistries, using optical microscopy and a technique called “quartz crystal microbalance with energy dissipation (QCM-D) monitoring.”
Dr. Hayashi explains, “We chose QCM-D because it is an ultrasensitive device that can monitor adsorbed masses as small as nanograms using acoustic waves. Similarly, SAMs with tunable terminal groups functionalized on atomically flat QCM sensors were chosen since they can help untangle the influence of surface chemistry from that of substrate topography on bacterial attachment.”
Studies have revealed that the standard mass loading explanation falls short of providing a complete account of bacterial adhesion to a surface. This is because the theory fails to account for the considerable effects on biofilm formation of the positive and negative shifts in the resonant frequency (f) brought on by the adsorption of extra mass onto the surface.
Considering this complex behavior through a coupled-resonator model, the team examined the changes in f and the energy dissipation (D) of the QCM sensor oscillation and assessed their impact on bacterial attachment and viscoelasticity of the SAM surfaces with different functionalities.
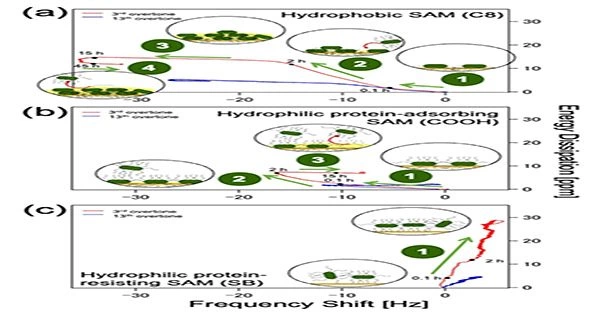
Figure 1: Plot of energy dissipation(D) vs shift in resonant frequency (f) for hydrophobic and hydrophilic protein-adsorbing SAMs and hydrophilic protein-resisting SAM at n =3 (3rd overtone, red) and n = 13 (13th overtone, blue) to understand time progression of bacterial adhesion.
The microscopy images along with QCM-D data revealed that E. coli adhered firmly to both hydrophobic (methyl-terminated) and hydrophilic protein-adsorbing (amine and carboxy-terminated) SAMs (Figure 1a and b), forming dense bacterial films. In contrast, it attached weakly to hydrophilic protein-resisting SAMs (Figure 1c), such as oligo (ethylene glycol) and sulfobetaine, and formed sparse and dissipative biofilms.
The scientists also noticed a positive f shift for SAMs that were resistant to hydrophilic proteins, probably because E. coli’s bacterial appendages played a part in both the initial contact and bonding with the surface as well as the creation of elastic linkages that resembled springs.
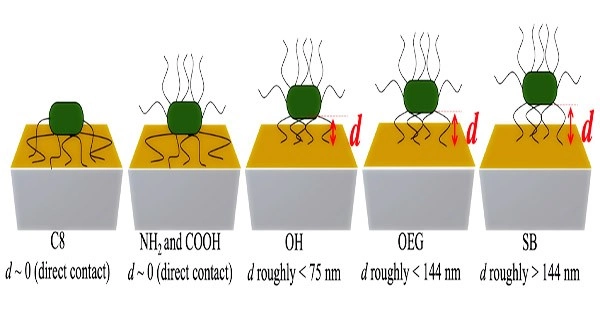
Figure 2: By estimating the acoustic wave penetration depths at each overtone, the distances of E. coli cells from each SAM surface could be determined, providing a clue on what makes bacterial cells adhere strongly to some surfaces and only weakly to others.
They also offered a potential explanation for why bacterial cells stick firmly to some surfaces but weakly to others by measuring the distances of the bacterial cell bodies from various SAM surfaces based on the various acoustic wave penetration depths at each overtone (Figure 2).
These insightful observations could aid in identifying surfaces that are more likely to be contaminated by biofilm and pave the way for contamination-resistant surfaces.
“Our study can act as practical guide for determining which surfaces are prone to bacterial fouling as well as for designing prevention strategies such as bacteria-resistant surfaces and antibiofouling coatings,” concludes Dr. Hayashi.