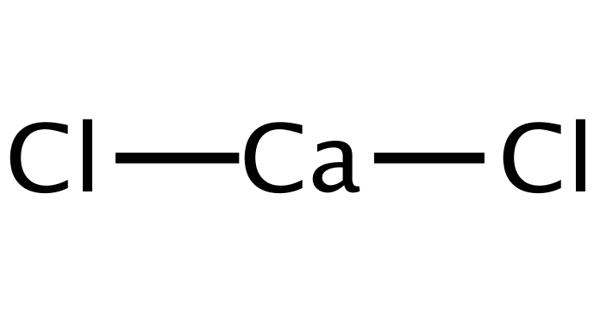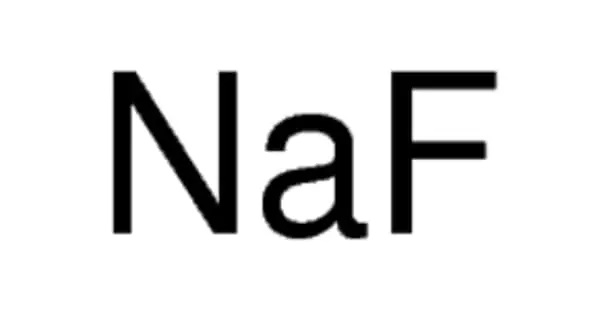At normal atmospheric pressure and temperatures less than 54.36 K (−218.79 °C, −361.82 °F), solid oxygen develops. Solid oxygen O2 is a clear solid with a light sky-blue color generated by absorption in the red region of the visible light spectrum, similar to liquid oxygen.
Solid oxygen is a one-of-a-kind crystal that combines the qualities of a simple molecular solid and a magnet. In contrast to typical magnets, the exchange interaction in solid oxygen operates against a backdrop of weak Van der Waals forces, supplying a large portion of the total lattice energy. As a result, the magnetic and lattice characteristics of solid oxygen are inextricably linked, resulting in a tremendously rich phase diagram and multiple anomalies in thermal, magnetic, and optical properties.
The link between molecular magnetization and crystal structures, electrical structures, and superconductivity has piqued the interest of oxygen molecules. Oxygen is the only simple diatomic molecule with a magnetic moment (and one of the few molecules in general). This makes solid oxygen especially intriguing since it is a “spin-controlled” crystal with antiferromagnetic magnetic order in the low temperature phases. The magnetic characteristics of oxygen have been extensively researched.

Solid oxygen converts from an insulating to a metallic state under very high pressures, and at very low temperatures, it even transforms to a superconducting state. Solid oxygen structural studies began in the 1920s, and six different crystallographic phases are now firmly recognized. Solid oxygen has a density ranging from 21 cm3/mol in the -phase to 23.5 cm3/mol in the γ-phase.
It appears surprising that, whereas liquid hydrogen or nitrogen can be easily converted to solid form by evaporating the respective liquids to exhaustion, simple air pumps fail to effect the transition of state in the case of liquid oxygen. This is owing to the lower vapour pressure of solid oxygen at its melting point when compared to hydrogen or nitrogen under identical conditions, as well as the greater requirement for extremely precise heat isolation in the experiment’s setup.
The transition from liquid to solid can be easily accomplished by using charcoal as a gaseous condensing agent at low temperatures in conjunction with the use of appropriate vacuum vessels. Pure liquid oxygen in a well isolated vessel is subjected to the exhaust created by a quantity of charcoal held at about the temperature of boiling oxygen, and its pressure is reduced sufficiently to achieve solidification to a clear jelly.