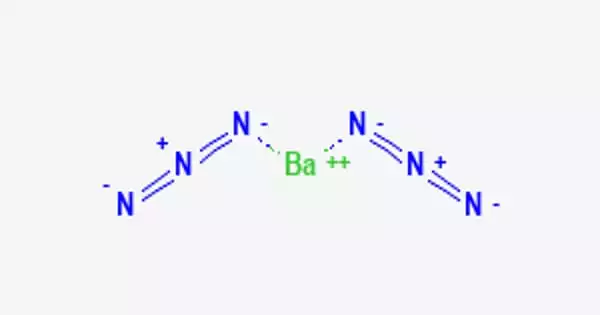
Barium azide, often known as Ba(N3)2, is an inorganic azide having the formula Ba(N3)2. It can be used as a getter for low luminescence and can increase the life of the filament several times. It is a crystalline solid that is combustible and can be utilized or transported in solution. It is explosive, as are most azides. It has a lower mechanical shock sensitivity than lead azide.
Barium azide appears as a white crystal slurry. Despite its water content, it is classified as a flammable solid. When dry, a powerful explosive ignites quickly and burns violently.
Properties
When shocked or heated, barium azide, a crystalline solid containing at least 50% water by mass, explodes. At 240°F (115.5°C), barium azide decomposes and emits nitrogen, and it is soluble in water. 1571 is the four-digit UN identifying number for barium azide. Its principal application is in high explosives.
- Appearance: Crystalline Solid
- Melting Point: 120ºC
- Molar Mass: 221.367 g/mol
- Boiling point: explodes
- Density: 2.936
- Form: monoclinic crystals
- Color: Monoclinic prisms or crystals

Preparation
By reacting sodium azide with a soluble barium salt, barium azine can be made. Because crystals will explode if fully dry or subjected to friction, the solution is concentrated to allow crystals to grow. The product should be kept moist and ethanol-free. To allow crystals to grow, the solution is concentrated. If crystals are totally dried or subjected to friction, they will burst. The product should be kept wet with ethanol.
Uses
Barium azide can be used to make azides of magnesium, sodium, potassium, lithium, rubidium and zinc with their respective sulfates.
Ba(N3)2 + Li2SO4 → 2LiN3 + BaSO4
It can also be used as a source for high pure nitrogen by heating:
Ba(N3)2 → Ba + 3N2
This reaction liberates metallic barium, which is used as a getter in vacuum applications.
Health Hazard
Some are poisonous, and if inhaled, eaten, or absorbed through the skin, they can be lethal. Skin and eye burns may result from contact. Fire can emit irritant, corrosive, and/or poisonous gases. Pollution may occur as a result of runoff from firefighting or diluting water.