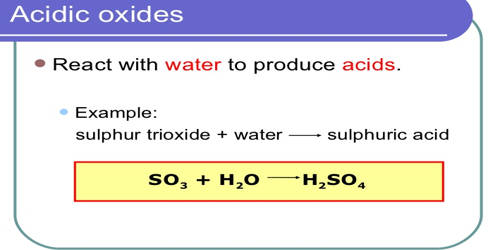
An oxide is a binary compound that we obtain upon the reaction of oxygen with other elements. Acidic oxide is an oxide that reacts with water to form an acid, or with a base to form a salt. It reacts with water and produces acid. They are oxides of either nonmetal or of metals in high oxidation states. Usually, it is the oxide of non-metals. Their chemistry can be systematically understood by taking an oxoacid and removing water from it until only an oxide remains. Non-metals react with oxygen to form acidic compounds of oxides which are held together by covalent bonds. The resulting oxide belongs to this group of substances. These compounds can also be called as acid anhydrides. For example, sulfurous acid (SO2), sulfuric acid (SO3), and carbonic acid (CO2) are acidic oxides. Acid anhydrides usually have a low melting and boiling point except for compounds like B2O3 and SiO2 which have high melting points and form giant molecules. An inorganic anhydride (a somewhat archaic term) is an acid anhydride without an organic moiety.
Sulfur dioxide reacts with water and gives sulfurous acid.
- SO2 + H2O → H2SO3
Acidic oxides are not Brønsted–Lowry acids because they do not donate protons; however, they are Arrhenius acids because they increase the hydrogen ion concentration of water. For instance, carbon dioxide increases the hydrogen ion concentration of rainwater (pH = 5.6) by a factor of 25 compared to pure water (pH = 7). They are also Lewis acids because they accept electron pairs from some Lewis bases, most notably base anhydrides.
The oxides of period three elements demonstrate periodicity with respect to acidity. As one moves across the period, the oxides become more acidic. Boric acid and carbonic acid are weak while the oxides of N, S, and the halides form the very strong acids nitric, sulfuric, perchloric. Sodium and magnesium oxides are alkaline. Aluminum oxides are amphoteric (reacting both as a base or acid). If the reaction with water produces a moderately acidic oxoacid, the oxide may or may not be soluble Silicon, phosphorus, sulfur, and chlorine oxides are acidic. Some non-metal oxides, such as a nitrous oxide (N2O) and carbon monoxide (CO), do not display any acid/base characteristics. If the oxoacid generated is weakly acidic, the oxide is usually but not always insoluble in water.
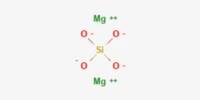
Acidic oxides can also react with basic oxides to produce salts of oxoanions:
- 2 MgO + SiO2 → Mg2SiO4
Acidic oxides are environmentally relevant. Sulfur and nitrogen oxides are considered air pollutants as they react with atmospheric water vapor to produce acid rain. Water-insoluble oxides are classed as acidic if they react with bases to form salts.