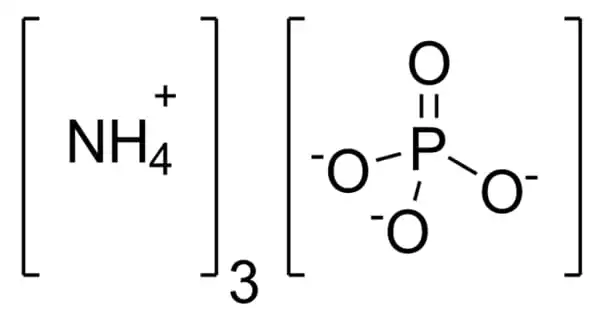
Ammonium phosphate is an inorganic chemical having the formula (NH4)3PO4. It’s the orthophosphoric acid ammonium salt. (NH4)3PO4 is a similar “double salt.” (NH4)2HPO4 is also recognized, however, it is impractical to employ. Both triammonium salts produce ammonia. In contrast to the unstable nature of triammonium salts, diammonium phosphate (NH4)2HPO4 and monoammonium salt (NH4)H2PO4 are stable compounds that are extensively employed as fertilizers to provide plants with fixed nitrogen and phosphorus.
Ammonium phosphate is a high source of elemental nitrogen that is utilized as a component in certain fertilizers. This is also utilized in thermoplastic formulations as a flame retardant. It adds nitrogen and phosphates to lawns that are deficient in these nutrients. Ammonium phosphate is a fast-release fertilizer that can be used for new grass planting, cleaning, monitoring, or lawn renovation.
Properties
- Density: 1.619 g/cubic cm
- Molecular Weight/ Molar Mass: 149.09 g/mol
- Boiling Point: 130°C
- Melting Point: 155 °C (311 °F; 428 K) decomposes
- Odor: Ammonia odor
- Appearance: White, tetrahedral crystals

Chemical Properties
Ammonium phosphate readily undergoes decomposition reaction emitting very toxic fumes. It forms phosphoric acid and ammonia.
(NH4)3PO4 → 3NH3 + H3PO4
Ammonium phosphate reacts with lead nitrate forming lead phosphate and ammonium nitrate.
4(NH4)3PO4 + 3Pb(NO3)4 → Pb3(PO4)4 + 12NH4NO3
Preparation
Triammonium phosphate can be prepared in the laboratory by treating 85% phosphoric acid with 30% ammonia solution:
H3PO4 + 3 NH3 → (NH4)3PO4
(NH4)3PO4 is a colorless, crystalline solid. The solid, which has the odor of ammonia, is readily soluble in water. The salt converts to diammonium hydrogen phosphate (NH4)2HPO4.
It is created by combining ammonium phosphate and urea in a molten state. Significant heat is generated, causing the ammonium phosphate sulphate to become molten. It is a class of nitrogen phosphorus compounds that comprises monoammonium phosphates and diammonium phosphates, as well as mixes of the two and combinations with ammonium nitrate or ammonium sulfate.
Uses
- Ammonium phosphate is a broad general term for a range of nitrogen and phosphate-containing fertilizer products.
- Primarily used as a solid fertilizer, but it can also be used in a solution.
- As an acid catalyst, they are used as a component of intumescent paints and mastics.
- Used in paints that contain pentaerythritol as a carbonific component and melamine as a particular ingredient.