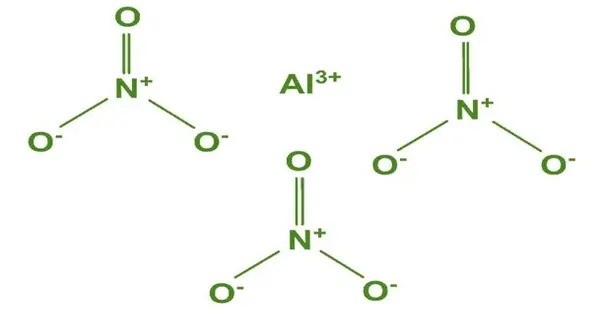
Aluminium nitrate is a white, water-soluble salt of aluminium and nitric acid, most commonly existing as the crystalline hydrate, aluminium nitrate nonahydrate, Al(NO3)3·9H2O. t is typically found as a white crystalline solid. It is highly soluble in water. It is an oxidizing agent due to the nitrate groups and can be reactive under certain conditions. It is mainly used in water treatment, in the manufacture of other aluminium compounds, and sometimes as a nitrating agent in organic synthesis.
Properties
- Chemical formula: Al(NO3)3
- Molar mass: 212.996 g/mol (anhydrous), 375.134 g/mol (nonahydrate)
- Appearance: White crystals, solid hygroscopic
- Odor: odorless
- Density: 1.72 g/cm3 (nonahydrate)
- Melting point: 66 °C (151 °F; 339 K) (anhydrous), 73.9 °C (165.0 °F; 347.0 K) (nonahydrate)
- Boiling point: 150 °C (302 °F; 423 K) (nonahydrate) decomposes
- Solubility in water: anhydrous: 60.0 g/100ml (0°C); 160 g/100ml (100 °C)
- nonahydrate: 67.3 g/100 mL
- Solubility in methanol: 14.45 g/100ml
- Solubility in ethanol: 8.63 g/100ml
- Solubility in ethylene glycol: 18.32 g/100ml
Preparation
Aluminium nitrate cannot be synthesized by the reaction of aluminium with concentrated nitric acid, as the aluminium forms a passivation layer. It may instead be prepared by the reaction of nitric acid with aluminium(III) chloride. Nitrosyl chloride is produced as a by-product; it bubbles out of the solution as a gas. More conveniently, the salt can be made by reacting nitric acid with aluminium hydroxide.
Aluminium nitrate may also be prepared a metathesis reaction between aluminium sulfate and a nitrate salt with a suitable cation such as barium, strontium, calcium, silver, or lead. e.g. Al2(SO4)3 + 3 Ba(NO3)2 → 2 Al(NO3)3 + 3 BaSO4.
Aluminium nitrate is typically prepared by reacting aluminium hydroxide or aluminium oxide with nitric acid:
Al(OH) 3+3HNO3 →Al(NO3)3 +3H2O
It cannot be made by direct reaction of aluminium metal with nitric acid, because it forms a passive oxide layer that prevents further reaction.
Natural Occurrence
Aluminium nitrate does not occur naturally in large quantities. This is due to aluminium’s tendency to form insoluble hydroxides or oxides in the presence of water, making nitrates unstable in nature.
Laboratory/Industrial Preparation:
Typically synthesized rather than mined.
From aluminium hydroxide or aluminium oxide:
𝐴𝑙(𝑂𝐻)3 + 3𝐻𝑁𝑂3 →𝐴𝑙(𝑁𝑂3)3 + 3𝐻2𝑂
𝐴𝑙2𝑂3 + 6𝐻𝑁𝑂3 → 2𝐴𝑙(𝑁𝑂3)3 +3𝐻2𝑂
Direct reaction of aluminium metal with nitric acid:
2Al+6HNO3 →2Al(NO3)3 +3H2 ↑
(Requires careful handling due to hydrogen evolution.)
Safety & Handling
- Oxidizer: Can enhance combustion of other materials.
- Irritant: Can cause skin, eye, and respiratory irritation.
- Decomposition: On heating, releases toxic gases like nitrogen oxides (NOₓ).
Uses
Aluminium nitrate is a strong oxidizing agent. It is used in tanning leather, antiperspirants, corrosion inhibitors, extraction of uranium, petroleum refining, and as a nitrating agent.
- As a laboratory reagent
- In nitration reactions and organic synthesis
- In tanning leather
- As a corrosion inhibitor
- In ceramics and textile processing