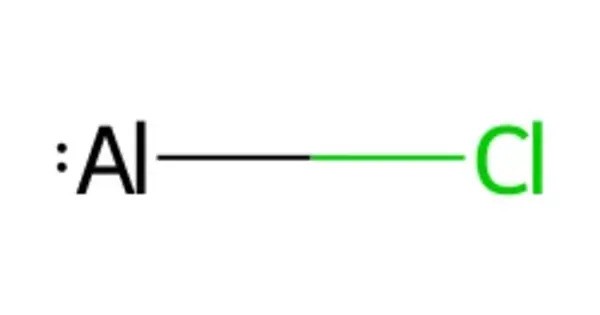
Aluminium monochloride, or chloridoaluminium is the metal halide with the formula AlCl. It is an inorganic compound and an intermediate species typically encountered in high-temperature chemistry involving aluminium and chlorine. Aluminium monochloride as a molecule is thermodynamically stable at high temperature and low pressure only. It’s unstable at room temperature, which limits its practical use and makes it primarily of interest in theoretical studies, high-temperature metallurgy, and gas-phase reactions.
This compound is produced as a step in the Alcan process to smelt aluminium from an aluminium-rich alloy. When the alloy is placed in a reactor that is heated to 1,300 °C and mixed with aluminium trichloride, a gas of aluminium monochloride is produced.
2Al(alloy) + AlCl3(gas) -> 3AlCl(gas)
It then disproportionates into aluminium melt and aluminium trichloride upon cooling to 900 °C.
This molecule has been detected in the interstellar medium, where molecules are so dilute that intermolecular collisions are unimportant.
Properties
- Chemical formula: AlCl
- Molar mass: 62.43 g·mol−1
- Oxidation State of Al: +1
- State at Room Temp.: Not stable; exists at high temperatures
- Structure: Diatomic molecule (Al–Cl) in the gas phase
- Bond Type: Covalent
- Polarity: Polar molecule
Stability and Conditions of Formation
AlCl is unstable at room temperature, quickly disproportionating into:
2AlCl → Al + AlCl3
Forms at high temperatures (above ~1000°C), particularly in the gas phase during:
Chemical vapor deposition (CVD)
Metallurgical processes (e.g., refining aluminium with chlorine)
Occurrence
Not naturally occurring in any mineral form. Found transiently in:
- Aluminium smelting (e.g., Hall–Héroult process under specific conditions)
- High-temperature exhaust gases of rockets or flame synthesis
- Laboratory synthesis during reactions involving aluminium metal and chlorine gas at elevated temperatures.
Applications
Due to its instability, AlCl has no direct commercial uses, but it’s important in:
- High-temperature kinetics studies
- CVD processes for thin-film materials
- Understanding disproportionation reactions in aluminium chemistry