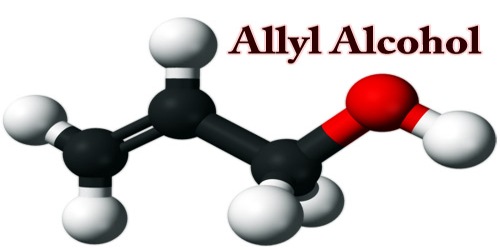
Allyl alcohol (IUPAC name: prop-2-en-1-ol) is an organic compound; it is a flammable, colorless liquid. It is an industrially important olefinic alcohol, and it’s structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colorless liquid. It is more toxic than typical small alcohols.
Allyl alcohol is the raw material for the synthesis of a wide range of allylic derivatives such as allyl diglycol carbonate, allyl glycidyl ether, 1,4-butanediol, poly(styrene-allyl alcohol), etc. These derivatives find application as lens, coupling agents, plasticizers, cross linking agents, and coating additives. Allyl alcohol can be prepared by the rhenium catalyzed-deoxydehydration of glycerol.
Allyl alcohol has a pungent, mustard-like odor. It is used in making drugs, organic chemicals, pesticides, in the manufacture of allyl esters, and as monomers and prepolymers for the manufacture of resins and plastics. It has a large use in the preparation of pharmaceutical products, in organic synthesis, and as a fungicide and herbicide. Occupational workers engaged in industries such as the manufacture of drugs, pesticides, allyl esters, organic chemicals, resins, war gas, and plasticizers, are often exposed to this alcohol.
Allyl alcohol is the smallest representative of the allylic alcohols. Allyl alcohol is mainly converted to glycerol, a reaction that proceeds via the intermediacy of glycidol. Otherwise a variety of polymerizable esters are prepared from allyl alcohol. It can be obtained by many methods. It was first prepared in 1856 by Auguste Cahours and August Hofmann by hydrolysis of allyl iodide. Today allyl alcohol is produced commercially by the Olin and Shell corporations through the hydrolysis of allyl chloride:
CH2=CHCH2Cl + NaOH → CH2=CHCH2OH + NaCl
Allyl alcohol can also be made by the rearrangement of propylene oxide, a reaction that is catalyzed by potassium alum at high temperatures. The advantage of this method relative to the allyl chloride route is that it does not generate salt. Also avoiding chloride-containing intermediates is the “acetoxylation” of propylene to allyl acetate:
CH2=CHCH3 + 1/2 O2 + CH3COOH → CH2=CHCH2OCOCH3 + H2O
Hydrolysis of this acetate gives allyl alcohol. In alternative fashion, propylene can be oxidized to acrolein, which upon hydrogenation gives the alcohol.
Allyl alcohol is prepared by several different processes; the original is alkaline hydrolysis of allyl chloride by steam injection at high temperatures. A more recent commercial process used oxidation of propylene to acrolein, which in turn reacts with secondary alcohol to yield allyl alcohol and a ketone. In this process, allyl alcohol is not isolated, but its aqueous stream is converted directly to glycerol. The most recent commercial process is the isomerization of propylene oxide over a lithium phosphate catalyst.
In principle, allyl alcohol can be obtained by dehydrogenation of propanol. In the laboratory, it has been prepared by the reaction of glycerol with oxalic or formic acids. Allyl alcohols in general can be prepared by allylic oxidation of allyl compounds by selenium dioxide.
Allyl alcohol’s flashpoint 70°F. Very toxic by inhalation and ingestion. Less dense than water (7.1 lb/gal). Vapors are heavier than air. Prolonged exposure to low concentrations or short exposure to high concentrations may have adverse health effects from inhalation. Allyl alcohol is a propenol in which the C=C bond connects C-2 and C-3. It is has been found in garlic (Allium sativum). Formerly used as a herbicide for the control of various grass and weed seeds. It has a role as an insecticide, a herbicide, an antibacterial agent, a fungicide, and a plant metabolite. It is a primary allylic alcohol and a propenol.
Allyl alcohol’s physical properties – it is colorless, mobile liquid with pungent, mustard-like odor at high concentrations. At low concentrations, odor resembles that of ethyl alcohol. Katz and Talbert (1930) and Dravnieks (1974) reported experimental detection odor threshold concentrations of 3.3 mg/m3 (1.4 ppmv) and 5 mg/m3 (2.1 ppmv), respectively.
Allyl alcohol may be used in the preparation of propylene via photocatalytic transfer hydrogenolysis in the presence of Pd/TiO2 catalysts. Allyl alcohol was used to grow fitter yeast (Saccharomyces cerevisiae). It is used to produce glycerol and acrolein and other allylic compounds. It is also used in the manufacture of military poison gas. The ester derivatives are used inresins and plasticizers. Allyl alcohol is also used in the production of transparent, decarbonate plastic used in scratch-resistant eyeglasses.
Allyl alcohol is a potent liver toxicant in laboratory animals following exposure by any route. Severe eye and respiratory tract irritation, decreased breathing rate, tremors, convulsions, diarrhea, coma, and lung and liver damage have been observed in laboratory animals exposed to moderate-to-high air levels of allyl alcohol. Severe skin irritation and pain, muscle spasms, liver and kidney damage, and blood in the urine were observed in laboratory animals after direct skin contact. Death has occurred in some animals after exposure to high levels via any route. No clear evidence of cancer was reported in laboratory animals following lifetime oral exposure to allyl alcohol.
Information Sources: