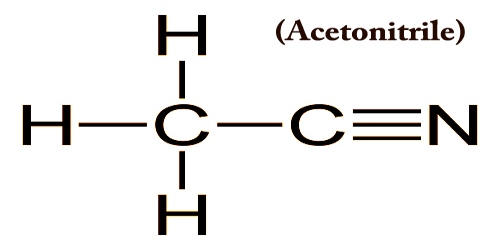
Acetonitrile is the chemical compound with the formula CH3CN, usually also called as nitrile methyl cyanide (MeCN) and methane. It appears as a colorless limpid liquid with an aromatic odor. Flashpoint 42°F. Density 0.783 g/cm3. The simplest organic nitrile is this colorless liquid (hydrogen cyanide is a simpler nitrile but the cyanide anion is not known as organic).
Acetonitrile is extremely explosive, with a peculiar scent like ether, and brightly burning flammable with fire. It has a role as a polar aprotic solvent and an EC 3.5.1.4 (amidase) inhibitor. For water, methanol, carbon tetrachloride, methyl acetate, ethyl acetate, ethylene dichloride, and many other non-saturated hydrocarbon solvents it is mutually soluble. This is an aliphatic nitrile and an organic compound that is volatile. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas.
Acetonitrile is primarily produced as a byproduct of the manufacture of acrylonitrile. It is poisonous and can be metabolized into cyanide and thiocyanate hydrogen. It is used in organic synthesis and in the purification of butadiene as a polar aprotic solvent. The N≡C−C skeleton is linear with a short C≡N distance of 1.16 Å. It is an important industrial solvent primarily used for the medium of organic synthesis (e.g. acetophenone, 1-naphthyl acetic acid, thiamine, etc.), extracting agent of fatty acids, and alcohol denaturant.
Acetonitrile ‘s main use is as a solvent that may be used because of the solvents used to synthesize fat-soluble vitamin, cortisone, carbon amine drugs, and their intermediate solvents. This is used as a medium-polarity solvent within the laboratory that is miscible with water and organic solvent dispersed, but not saturated hydrocarbons. Acetonitrile is also used in the processing of vitamin B1 and amino acids as an active medium-solvent. It has a sufficient spectrum of liquids and a high dielectric constant of 38.8. With a dipole moment of 3.92 D, acetonitrile dissolves a wide range of ionic and nonpolar compounds and is useful as a mobile phase in HPLC and LC-MS.
Acetonitrile can substitute chlorinated solvents as a vinyl coating, an extracting agent of carboxylic acid, an alcohol denaturant, the extracting agent of butadiene, and therefore the solvent of acrylonitrile synthetic fibers. Its ultraviolet transparency UV cutoff, low viscosity, and low chemical reactivity make it a preferred choice for high-performance liquid chromatography (HPLC). This also features many uses in the manufacture of fabric dyeing, light manufacturing, spice processing, and photographic materials. Acetonitrile is a popular two-carbon building block of many useful chemicals in organic synthesis, including acetamidine hydrochloride, thiamine, and α-napthaleneacetic acid.
A mixture of acetonitrile and sulfuric acid on heating (or self-heating) to 53 °C underwent an uncontrollable exothermic reaction to 160 °C in a few seconds. Acetonitrile is a by-product of acrylonitrile processing, and its processing has also declined, further compounding the scarcity of acetonitrile. Exposure to liquid or vapor can cause poisoning during the manufacturing process. Acetonitrile has only modest toxicity in small doses; generally, the onset of toxic effects is delayed, due to the time required for the body to metabolize acetonitrile to cyanide (generally about 2–12 hours).
Although acetonitrile causes symptoms such as nausea, vomiting, stomach pain, diarrhea, chest pain, exhaustion, and weakness compared to hydrogen cyanide, and respiratory depression in extreme cases, it often also triggers hypotension, coma, and convulsions, but its onset cycle is relatively slow with an incubation period above 4H; nor does it cause disease as serious as hydrogen cyanide. It also rarely causes sudden death; for poisoned patients, their heart rates, pulse rates still because the respiration rates decrease. The treatment is as for poisoning, with oxygen, nitrite, and hypo among the foremost commonly used emergency treatments. Acetonitrile has been employed in formulations for cosmetic remover, despite its toxicity. Furthermore, it’s also applied in the recrystallization of steroids and extraction of carboxylic acid and also used because of the solvents of high liquid chromatography (HPLC).
Information Sources: