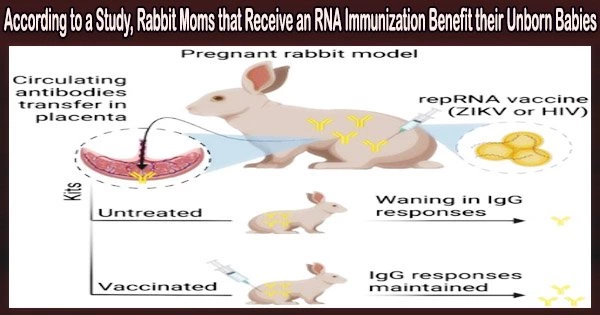
Researchers report in the journal Molecular Therapy that newly created mRNA vaccines against the Zika virus and HIV-1 induced potent antibody responses that passed from pregnant rabbits to their offspring.
The scientists state that the findings justify future research and development of their LION/repRNA vaccination platform for use in maternal and neonatal settings to prevent the transmission of infections from mother to child in both humans and animals.
The development of mRNA vaccines for additional infectious diseases is sped up by the recent success of mRNA vaccines in combating the COVID-19 pandemic. The U.S. Food and Drug Administration has approved mRNA vaccinations for children 6 months of age and older, and early research in expectant mothers has not revealed any clear harm.
“Preventing mother-to-child transmission is a major goal for reducing disease burden in newborns,” says senior author Amit Khandhar, a material scientist at HDT Bio Corp. “With mRNA vaccines attracting global attention, there is a need to evaluate their safety and immunogenicity in preclinical models that inform maternal and childhood vaccination.”
Khandhar collaborated with Herman Staats of Duke University School of Medicine and Noah Sather of Seattle Children’s Research Institute to evaluate self-amplifying replicon (repRNA) vaccines.
For instance, the immunization intervals we used were not optimized and will likely not translate to humans due to differences in the gestation periods between rabbits and humans. Further studies will be needed to test boosting intervals and the durability of antibody responses to maximize passive antibody transfer to newborns. Lastly, additional studies designed to measure safety signals in maternal and neonatal models will be needed before advancing to clinical evaluation.
Amit Khandhar
Using the Zika virus and HIV-1 as model disease targets, the researchers administered the vaccinations using their clinical-stage LION nanoparticle formulation in pregnant rabbits. Following mother-to-child transmission, these two microorganisms significantly contribute to the development of illnesses in infants.
The repRNA vaccines contain viral enzymes that, in comparison to non-replicating mRNA, increase the expression of a target gene by 10- to 100-fold. This has benefits for manufacturing and dosing.
In contrast to lipid nanoparticle formulations, which encase RNA, the patented LION delivery method is a stable oil-in-water nanoparticle emulsion that electrostatically binds and shields nucleic acids.
Because LION is stored independent of repRNA, it has plug-and-play functionality, allowing for rapid evaluation of new repRNA vaccine constructs such as those recently developed to address emerging SARS-CoV-2 variants.
The outcomes demonstrated that relatively high doses of repRNA immunization were well tolerated and had no negative effects on litter size. Strong antigen-specific antibody responses were also produced by the LION/repRNA vaccinations in adult pregnant rabbits, which most likely passively transmitted to offspring in utero.
“While the strong correlation in both the magnitude and quality of antibody levels between mothers and newborns suggests that the antibodies detected in kits were likely acquired passively from mothers, we cannot completely rule out the possibility that the vaccine administered to mothers may itself distribute to kits and actively induce antibody responses,” Khandhar says.
The timing of the mother’s immunization was also found to be crucial for maximizing the transfer of antibodies, and subsequent vaccination of babies maintained higher antibody levels than no vaccination.
Active immunization of neonates may be necessary for sustaining total antibody responses in children after birth in addition to optimal maternal vaccination timing. If RNA-based maternal vaccinations can provide protection against infection by mother-to-child transmission, more research is required, according to the scientists.
“For instance, the immunization intervals we used were not optimized and will likely not translate to humans due to differences in the gestation periods between rabbits and humans,” Khandhar says.
“Further studies will be needed to test boosting intervals and the durability of antibody responses to maximize passive antibody transfer to newborns. Lastly, additional studies designed to measure safety signals in maternal and neonatal models will be needed before advancing to clinical evaluation.”