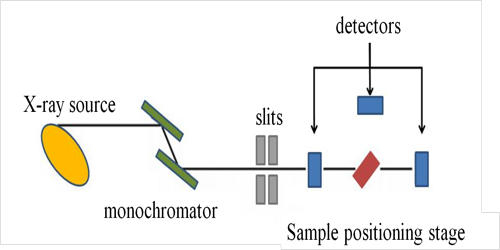
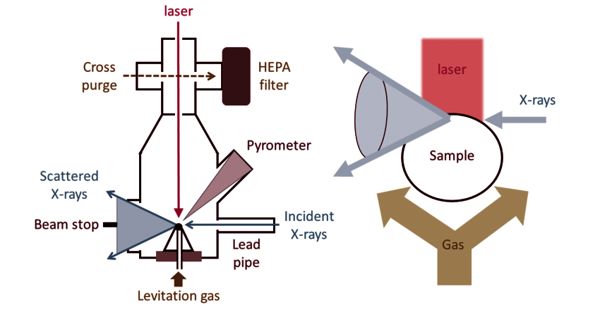
X-ray absorption spectroscopy (XAS) is a widely used technique for determining the local geometric and/or electronic structure of matter. It is a technique that provides a bulk measurement of the elemental and chemical composition in a specimen by virtue of its X-ray absorption characteristics. The experiment is usually performed at synchrotron radiation facilities, which provide intense and tunable X-ray beams. It is a powerful local-probe technique that provides structural information at the local level on magnetic nanocrystalline spinel-type materials. Samples can be in the gas phase, solutions, or solids. It is element-specific, so one can focus on one element without interference from other elements present in the sample. The more important aspect is that one can either trap intermediates in the enzymatic cycle or modify the site by the addition of inhibitors or substrate or generate other chemical modifications.
X-ray absorption spectroscopy was first used in the 1920s for structural investigations of the matter. Very generally, an X-ray strikes an atom and excites a core electron that can either be promoted to an unoccupied level or ejected from the atom. The significant advantage of XAS over the X-ray crystallography is that the local structural information around the element of interest can be obtained even from disordered samples, such as powders and solution. This theory has been developed to an extent that it can be applied to complicated molecules of known structure. It is not limited by the state of the sample, because it is sensitive only to the local metal site structure.
Applications
X-ray absorption spectroscopy (XAS) provides information about the electronic structure and the coordination environment of the elements in a sample. XAS is a technique used in different scientific fields including molecular and condensed matter physics, materials science and engineering, chemistry, earth science, and biology. It allows us to study the local structure of the element of interest without interference from absorption by the protein matrix, water, or air.
X-ray absorption spectroscopy is important in gaining structural understanding of a range of materials. Its use has increased due to the availability of synchrotron radiation facilities around the world. In particular, its unique sensitivity to the local structure, as compared to x-ray diffraction, have been exploited for studying:
- Amorphous solids and liquid systems
- Solid solutions
- Local distortions of crystal lattices
- Metalloproteins
- Metal clusters
- Catalysis
- Speciation of elements
- Liquid water and aqueous solutions
- Used to detect the bone fracture
- Used to determine the concentration of any liquid in any tank.