Valence bond theory assumes that electrons in a molecule are simply the electrons in the original atomic orbitals, with some used while bonding. In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that was developed to use the methods of quantum mechanics to explain chemical bonding. This Theory was developed in order to explain chemical bonding using the method of quantum mechanics. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds when a molecule is formed. This theory primarily focuses on the formation of individual bonds from the atomic orbitals of the participating atoms during the formation of a molecule. In contrast, the molecular orbital theory has orbitals that cover the whole molecule.
Theory
According to the valence bond theory, “Electrons in a molecule occupy atomic orbitals rather than molecular orbitals. The atomic orbitals overlap on the bond formation and the larger the overlap the stronger the bond.”
According to this theory, a covalent bond is formed between two atoms by the overlap of half-filled valence atomic orbitals of each atom containing one unpaired electron. The theory given by Lewis explained the structure of molecules. However, it failed to explain the chemical bond formation. A valence bond structure is similar to a Lewis structure, but where a single Lewis structure cannot be written, several valence-bond structures are used. Each of these VB structures represents a specific Lewis structure. This combination of valence bond structures is the main point of resonance theory. Valence bond theory considers that the overlapping atomic orbitals of the participating atoms form a chemical bond. The overlapping of atomic orbitals results in the formation of a chemical bond. Because of the overlapping, it is most probable that electrons should be in the bond region. The electrons are localized in the bond region due to overlapping.
The overlapping atomic orbitals can differ. The two types of overlapping orbitals are sigma and pi. Sigma bonds occur when the orbitals of two shared electrons overlap head-to-head. Pi bonds occur when two orbitals overlap when they are parallel. For example, a bond between two s-orbital electrons is a sigma bond, because two spheres are always coaxial. In terms of bond order, single bonds have one sigma bond, double bonds consist of one sigma bond and one pi bond, and triple bonds contain one sigma bond and two pi bonds.
Applications of this Theory
- The maximum overlap condition which is described by the valence bond theory can explain the formation of covalent bonds in several molecules.
- This is one of its most important applications. For example, the difference in the length and strength of the chemical bonds in H2 and F2 molecules can be explained by the difference in the overlapping orbitals in these molecules.
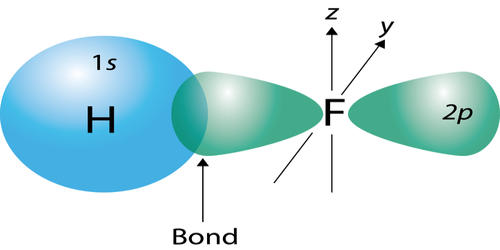
- The covalent bond in an HF molecule is formed from the overlap of the 1s orbital of the hydrogen atom and a 2p orbital belonging to the fluorine atom, which is explained by the valence bond theory.
Limitations of this Theory
The shortcomings of the valence bond theory include
- Failure to explain the tetravalency exhibited by carbon
- No insight offered on the energies of the electrons.
- The theory assumes that electrons are localized in specific areas.
- No distinction between weak and strong ligands.
- No explanation for the color exhibited by coordination compounds.