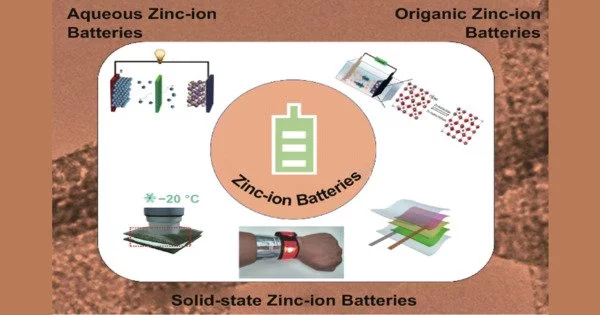
A zinc-carbon battery is a type of dry cell primary battery that generates direct electric current through the electrochemical reaction of zinc and manganese dioxide (MnO2) in the presence of an electrolyte. It generates a 1.5-volt voltage between the zinc anode, which is typically built as a cylindrical container for the battery cell, and a carbon rod surrounded by a compound with a higher standard electrode potential (positive polarity), known as the cathode, which collects the current from the manganese dioxide electrode. The name “zinc-carbon” is slightly misleading because it implies that carbon, rather than manganese dioxide, is the reducing agent.
While scientists hoped that rechargeable zinc-manganese dioxide batteries could be developed into a viable alternative for grid storage applications, engineers at the University of Illinois Chicago and their colleagues discovered the atomistic mechanism of charge and discharge in such batteries.
The scientists arrived at this conclusion after using advanced electron microscopy, electrochemical experiments, and theoretical calculations to investigate how the zinc anode interacts with the manganese cathode in the battery system. Their findings were published in Nature Sustainability.
We discovered that hydrogen is responsible for the damage to the manganese dioxide tunnel structures, further reducing the battery’s recharging potential. The results of these experiments provide important atomic insights into the mechanisms of the zinc-manganese battery. We now have a compass for developing better strategies because we understand what is going on at the cellular level.
Shahbazian-Yassar
“Zinc and manganese separately have very favorable properties for high-quality sustainable batteries; however, when paired in a full system their intercalation – their rechargeability – has been debatable, with some recent studies suggesting zinc insertion and deinsertion in manganese dioxide is responsible for the rechargeability of the cells,” said study lead author Reza Shahbazian-Yassar, UIC professor of mechanical and industrial engineering in the College of Engineering. “With this study, we showed there is actually no microscopic evidence of zinc reinsertion into manganese dioxide, and what was previously thought to be indicators of recharging was from positively charged hydrogen ions being inserted in the manganese, not zinc.”
The researchers built aqueous zinc-manganese dioxide cells and tested them over 100 cycles in their experiments. In experiments, they discharged and attempted to recharge the batteries while using electron microscopy to capture atomic-level images of the reactions.
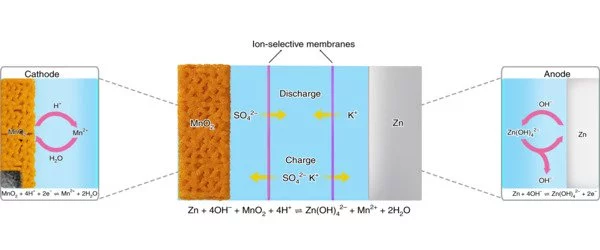
“We discovered that hydrogen is responsible for the damage to the manganese dioxide tunnel structures, further reducing the battery’s recharging potential,” Shahbazian-Yassar explained. “The results of these experiments provide important atomic insights into the mechanisms of the zinc-manganese battery. We now have a compass for developing better strategies because we understand what is going on at the cellular level.”
As an electrolyte, general-purpose batteries may use an acidic aqueous paste of ammonium chloride (NH4Cl), with some zinc chloride solution on a paper separator acting as a salt bridge. Heavy-duty types make use of a paste primarily made of zinc chloride (ZnCl2).
Zinc-carbon batteries were the first commercial dry batteries, derived from the wet Leclanché cell technology. Because the battery provided a higher energy density at a lower cost than previously available cells, they enabled flashlights and other portable devices. They’re still useful in low-power or intermittent-use devices like remote controls, flashlights, clocks, and transistor radios. Zinc-carbon dry cells are primary cells that are only used once. Today, zinc-carbon batteries have largely been replaced by more efficient and safe alkaline batteries.